Neonatal Fetal Nutrition & Metabolism
Category: Abstract Submission
Neonatal Fetal Nutrition & Metabolism I
279 - Randomized Trial of Early Enhanced Parenteral Nutrition and Later Neurodevelopment in Preterm Infants
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 279
Publication Number: 279.119
Publication Number: 279.119
Erin Morris, University of Minnesota Masonic Children's Hospital, Roseville, MN, United States; Neely Miller, University of Minnesota, Minneapolis, MN, United States; Nicholas A. Marka, University of Minnesota Medical School, Minneapolis, MN, United States; Juan D. Gonzalez Villamizar, Children’s Minnesota, Minneapolis, MN, United States; Sara E. Ramel, University of Minnesota Masonic Children's Hospital, North Oaks, MN, United States

Erin Morris, MD
Fellow
University of Minnesota Masonic Children's Hospital
Roseville, Minnesota, United States
Presenting Author(s)
Background: Early growth failure in preterm infants is associated with worse neurodevelopmental outcomes. Increased calories, protein, and lipid provision in the first week of life are associated with improved growth and neurodevelopment. These associations are not based on randomized trials and thus risk confounding by non-nutritional factors, eg, degree of illness.
Objective: To determine whether infants randomized to an enhanced parenteral nutrition protocol vs a standard nutrition protocol have improved developmental outcomes at 4 or 12 months corrected age (CA).
Design/Methods: Preterm infants ( < 32 weeks gestational age and < 1500 grams) were enrolled and randomized to receive an enhanced parenteral nutrition protocol during the first week of life or standard parenteral nutrition (control group). Infants in the enhanced group received a higher glucose infusion rate (GIR) (5.5 mgs/kg/min vs 4 mgs/kg/min) and were advanced more quickly (1.5 mgs/kg/min vs 1 mg/kg/min per day) to a similar maximum GIR of 12 mgs/kg/min. Infants in the enhanced group started intralipids at 2 grams/kg/day (vs 0.5 gms/kg/day); both groups advanced to a max of 3.5 grams/kg/day. Pattern-reversal visual evoked potentials assessing neural speed of processing were obtained at 4 month CA and Bayley Scales of Infant Development (BSID) assessed general outcome at 1 year CA.
Results: Of the 80 infants enrolled in the study, 34 infants had neurodevelopmental follow up at 4 months CA (18 control and 16 enhanced) and baseline demographics were similar. Average kcal/kg/day intake was not significantly different between the groups (102.8 vs. 111.4 kcal/kg/day, p-value = 0.082). In multivariable analysis, the P100 latency was longer in the intervention group, indicative of slower speed of processing (Table 2). Scores on the BSID were not significantly different at 1 year CA. Additionally, an analysis of kcal/kg/day (regardless of randomization group) did not show differences in visual evoked potentials.Conclusion(s): Enhanced early parenteral nutrition during the first week of life did not improve speed of processing at 4 months CA or developmental scores at 12 months CA in this small group of preterm infants. Low follow-up rates, secondary to the COVID-19 pandemic, limited the power of this study. Larger randomized trials are needed to investigate the impact of early parenteral nutrition on later development and to determine optimal caloric intake goals for preterm neonates in the first week of life.
Erin Morris CVE Morris CV.pdf
Multivariable VEP Results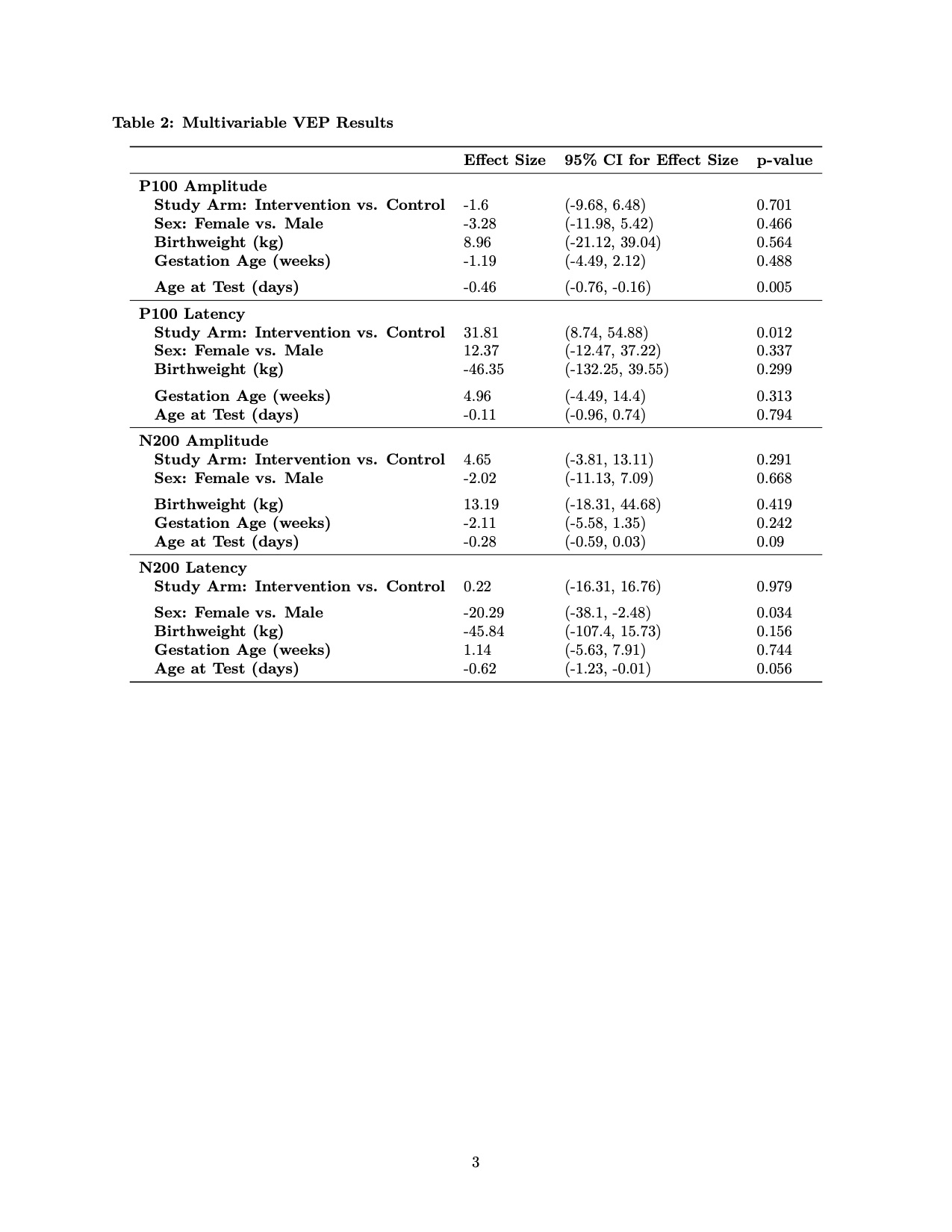 VEP- Visual Evoked Potential
VEP- Visual Evoked Potential
Objective: To determine whether infants randomized to an enhanced parenteral nutrition protocol vs a standard nutrition protocol have improved developmental outcomes at 4 or 12 months corrected age (CA).
Design/Methods: Preterm infants ( < 32 weeks gestational age and < 1500 grams) were enrolled and randomized to receive an enhanced parenteral nutrition protocol during the first week of life or standard parenteral nutrition (control group). Infants in the enhanced group received a higher glucose infusion rate (GIR) (5.5 mgs/kg/min vs 4 mgs/kg/min) and were advanced more quickly (1.5 mgs/kg/min vs 1 mg/kg/min per day) to a similar maximum GIR of 12 mgs/kg/min. Infants in the enhanced group started intralipids at 2 grams/kg/day (vs 0.5 gms/kg/day); both groups advanced to a max of 3.5 grams/kg/day. Pattern-reversal visual evoked potentials assessing neural speed of processing were obtained at 4 month CA and Bayley Scales of Infant Development (BSID) assessed general outcome at 1 year CA.
Results: Of the 80 infants enrolled in the study, 34 infants had neurodevelopmental follow up at 4 months CA (18 control and 16 enhanced) and baseline demographics were similar. Average kcal/kg/day intake was not significantly different between the groups (102.8 vs. 111.4 kcal/kg/day, p-value = 0.082). In multivariable analysis, the P100 latency was longer in the intervention group, indicative of slower speed of processing (Table 2). Scores on the BSID were not significantly different at 1 year CA. Additionally, an analysis of kcal/kg/day (regardless of randomization group) did not show differences in visual evoked potentials.Conclusion(s): Enhanced early parenteral nutrition during the first week of life did not improve speed of processing at 4 months CA or developmental scores at 12 months CA in this small group of preterm infants. Low follow-up rates, secondary to the COVID-19 pandemic, limited the power of this study. Larger randomized trials are needed to investigate the impact of early parenteral nutrition on later development and to determine optimal caloric intake goals for preterm neonates in the first week of life.
Erin Morris CVE Morris CV.pdf
Multivariable VEP Results
 VEP- Visual Evoked Potential
VEP- Visual Evoked Potential