Critical Care
Category: Abstract Submission
Critical Care I
19 - A Desirability of Outcome Ranking (DOOR) Scale: Development of a Novel Outcomes Scale for Pediatric Critical Illness
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 19
Publication Number: 19.102
Publication Number: 19.102
Grace E. Logan, Children's Hospital Colorado, Denver, CO, United States; Kristen Miller, University of Colorado School of Medicine, Aurora, CO, United States; Yamila L. Sierra, Children's Hospital Colorado, Denver, CO, United States; Peter M. Mourani, University of Arkansas for Medical Sciences, Little Rock, AR, United States; Tellen D. Bennett, University of Colorado School of Medicine, Aurora, CO, United States; Stephanie L. Bourque, University of Colorado School of Medicine, Greenwood Village, CO, United States; Aline B. Maddux, University of Colorado School of Medicine, Children's Hospital Colorado, Aurora, CO, United States
- GL
Grace E. Logan, MD
Pediatric Critical Care Fellow
Children's Hospital Colorado
Denver, Colorado, United States
Presenting Author(s)
Background: Adult critical care trials commonly use composite ordinal scale outcome measures, including the Desirability of Outcome Ranking (DOOR) scale. A similar scale for use in critically ill children does not exist. Additionally, the association between attainment of short-term outcomes commonly used in pediatric critical care medicine (PCCM) trials (e.g., duration of mechanical ventilation) with long-term outcomes, such as health-related quality of life (HRQL), has not been evaluated.
Objective: To evaluate a novel PCCM DOOR scale (Figure 1) applied at post-intubation day 7 and its association with 3-month post-discharge outcomes in a cohort of critically ill children.
Design/Methods: We developed and applied the PCCM DOOR scale to patients < 18 years-old with respiratory failure due to septic shock or parenchymal lung disease requiring invasive ventilation for > 72 hours. Fisher’s Exact and Wilcoxon Rank Sum tests were used to compare the proportion of patients with a functional decline (increase in Functional Status Scale [FSS] score), or a clinically significant worsening of HRQL (> 4.5 points decrease in the PedsQLTM score), or death at 3 months relative to pre-illness baseline. Due to small numbers in each group, comparisons were made between patients with DOOR ranking < 3 versus > 3 on post-intubation day 7.
Results: We evaluated 73 patients (50 [68%] with parenchymal lung disease and 23 [32%] with septic shock); median age was 1.4 years (interquartile range [IQR] 0.5, 9.6) (Table 1). At day 7, 1 patient had died, 28 (38%) required ongoing life-sustaining therapies, 27 (37%) were hospitalized with organ dysfunction, 9 (12%) were hospitalized without organ dysfunction, and 7 (10%) were discharged (Figure 2). At 3 months after discharge, most patients did not have a worsened functional status (n=44; 68%) or HRQL (n=37; 58%) (Figure 2). Of the 30 patients with DOOR scale < 3, 7 (23%) had incomplete follow-up data, 10 (40%) had functional decline at 3 months, and 9 (36%) had worsened HRQL (Figure 2). Of the 43 patients with a DOOR scale > 3, 4 (9%) patients had incomplete follow-up data, 11 (28%) had functional decline, and 18 (46%) had a worsened HRQL. The distribution of FSS and HRQL outcomes did not differ by dichotomized DOOR outcome groups. Conclusion(s): A higher proportion of patients with a DOOR score < 3 at 7 days experienced a functional decline compared to those with DOOR >3, although this difference did not reach statistical significance. Further evaluation in a larger cohort is important to determine the utility of this PCCM DOOR outcome scale.
Figure 1. Desirability of Outcome Ranking (DOOR) Scale defined for a PCCM cohort with respiratory failure due to parenchymal lung disease or septic shock.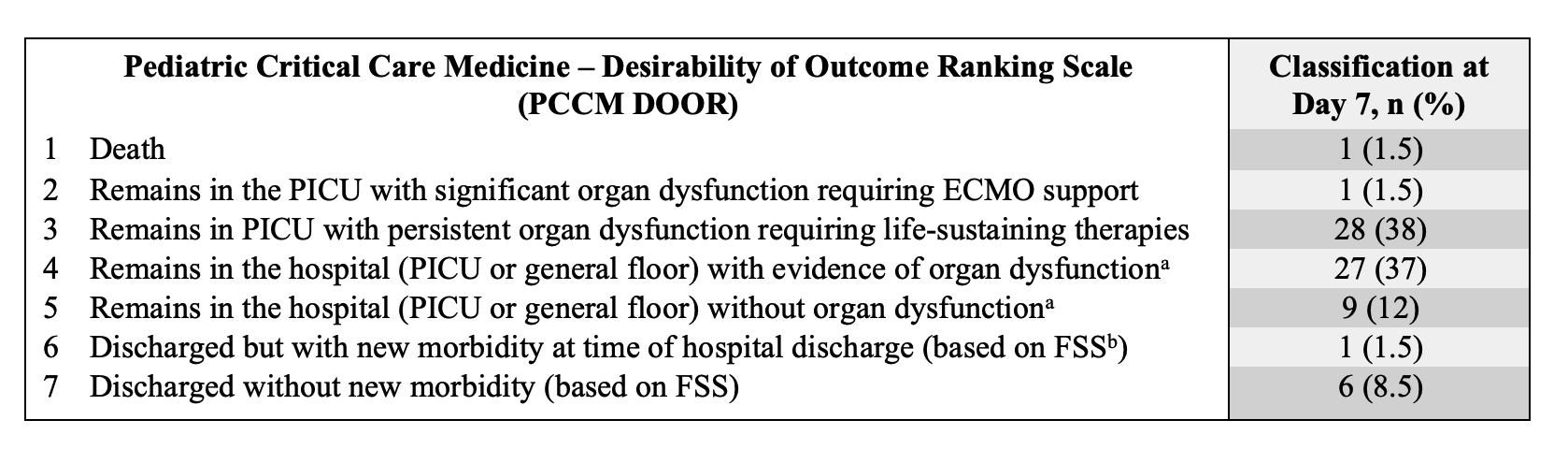 The scale was modified from the World Health Organization (WHO) Clinical Progression Scale, which is currently being used to assess outcomes in interventional trials in patients with respiratory failure due to SARS-CoV-2 infection.
The scale was modified from the World Health Organization (WHO) Clinical Progression Scale, which is currently being used to assess outcomes in interventional trials in patients with respiratory failure due to SARS-CoV-2 infection.
a We defined organ dysfunction as a Pediatric Logistic Organ Dysfunction-2 (PELOD-2) score ≥2.
b The Functional Status Scale (FSS) assigns a score and category of functional status (from normal to very severe) based on functional milestones across various domains, including mental status, sensory, communication, motor, feeding and respiratory.
PCCM: Pediatric Critical Care Medicine, PICU: Pediatric Intensive Care Unit, ECMO: Extracorporeal Membrane Oxygenation, FSS: Functional Status Scale
Table 1. Characteristics for a cohort (n = 73) of critically ill pediatric patients requiring mechanical ventilation for ≥72 hours.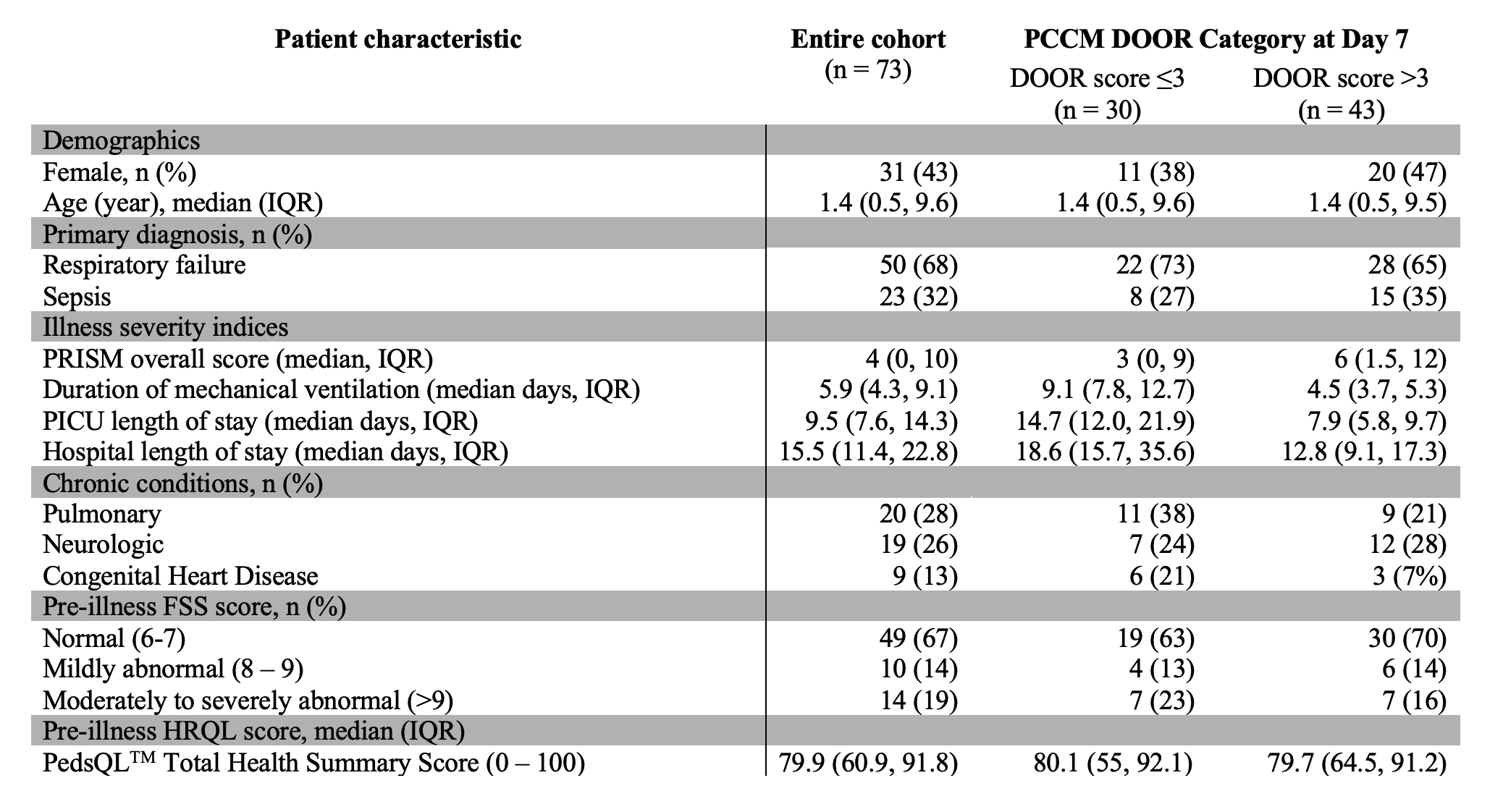 PICU: Pediatric Intensive Care Unit; PRISM: Pediatric Risk of Mortality Score; PCCM DOOR: Pediatric Critical Care Medicine Desirability of Outcome Ranking; IQR: Interquartile Range; FSS: Functional Status Scale
PICU: Pediatric Intensive Care Unit; PRISM: Pediatric Risk of Mortality Score; PCCM DOOR: Pediatric Critical Care Medicine Desirability of Outcome Ranking; IQR: Interquartile Range; FSS: Functional Status Scale
Objective: To evaluate a novel PCCM DOOR scale (Figure 1) applied at post-intubation day 7 and its association with 3-month post-discharge outcomes in a cohort of critically ill children.
Design/Methods: We developed and applied the PCCM DOOR scale to patients < 18 years-old with respiratory failure due to septic shock or parenchymal lung disease requiring invasive ventilation for > 72 hours. Fisher’s Exact and Wilcoxon Rank Sum tests were used to compare the proportion of patients with a functional decline (increase in Functional Status Scale [FSS] score), or a clinically significant worsening of HRQL (> 4.5 points decrease in the PedsQLTM score), or death at 3 months relative to pre-illness baseline. Due to small numbers in each group, comparisons were made between patients with DOOR ranking < 3 versus > 3 on post-intubation day 7.
Results: We evaluated 73 patients (50 [68%] with parenchymal lung disease and 23 [32%] with septic shock); median age was 1.4 years (interquartile range [IQR] 0.5, 9.6) (Table 1). At day 7, 1 patient had died, 28 (38%) required ongoing life-sustaining therapies, 27 (37%) were hospitalized with organ dysfunction, 9 (12%) were hospitalized without organ dysfunction, and 7 (10%) were discharged (Figure 2). At 3 months after discharge, most patients did not have a worsened functional status (n=44; 68%) or HRQL (n=37; 58%) (Figure 2). Of the 30 patients with DOOR scale < 3, 7 (23%) had incomplete follow-up data, 10 (40%) had functional decline at 3 months, and 9 (36%) had worsened HRQL (Figure 2). Of the 43 patients with a DOOR scale > 3, 4 (9%) patients had incomplete follow-up data, 11 (28%) had functional decline, and 18 (46%) had a worsened HRQL. The distribution of FSS and HRQL outcomes did not differ by dichotomized DOOR outcome groups. Conclusion(s): A higher proportion of patients with a DOOR score < 3 at 7 days experienced a functional decline compared to those with DOOR >3, although this difference did not reach statistical significance. Further evaluation in a larger cohort is important to determine the utility of this PCCM DOOR outcome scale.
Figure 1. Desirability of Outcome Ranking (DOOR) Scale defined for a PCCM cohort with respiratory failure due to parenchymal lung disease or septic shock.
 The scale was modified from the World Health Organization (WHO) Clinical Progression Scale, which is currently being used to assess outcomes in interventional trials in patients with respiratory failure due to SARS-CoV-2 infection.
The scale was modified from the World Health Organization (WHO) Clinical Progression Scale, which is currently being used to assess outcomes in interventional trials in patients with respiratory failure due to SARS-CoV-2 infection.a We defined organ dysfunction as a Pediatric Logistic Organ Dysfunction-2 (PELOD-2) score ≥2.
b The Functional Status Scale (FSS) assigns a score and category of functional status (from normal to very severe) based on functional milestones across various domains, including mental status, sensory, communication, motor, feeding and respiratory.
PCCM: Pediatric Critical Care Medicine, PICU: Pediatric Intensive Care Unit, ECMO: Extracorporeal Membrane Oxygenation, FSS: Functional Status Scale
Table 1. Characteristics for a cohort (n = 73) of critically ill pediatric patients requiring mechanical ventilation for ≥72 hours.
 PICU: Pediatric Intensive Care Unit; PRISM: Pediatric Risk of Mortality Score; PCCM DOOR: Pediatric Critical Care Medicine Desirability of Outcome Ranking; IQR: Interquartile Range; FSS: Functional Status Scale
PICU: Pediatric Intensive Care Unit; PRISM: Pediatric Risk of Mortality Score; PCCM DOOR: Pediatric Critical Care Medicine Desirability of Outcome Ranking; IQR: Interquartile Range; FSS: Functional Status Scale