Neonatal Quality Improvement
Category: Abstract Submission
Neonatal Quality Improvement V
398 - We are Colostrum Champions: A QI Initiative to Improve Early Colostrum Administration to VLBW Infants at a Level 3 NICU
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 398
Publication Number: 398.435
Publication Number: 398.435
Noa Fleiss, Yale School of Medicine, New Haven, CT, United States; Corinne Morrison, Yale-New Haven Children's Hospital, New Haven, CT, United States; Therese-Ann Deal, Yale New Haven Childrens Hospital, Trumbull, CT, United States; Allison Nascimento, Yale-New Haven Children's Hospital, Fairfield, CT, United States; Debra L. Stone, Yale-New Haven Children's Hospital, Fairfield, CT, United States; Eliza H. Myers, Yale New Haven Children's Hospital, NEW HAVEN, CT, United States

Noa Fleiss, MD (she/her/hers)
Assistant Professor of Pediatrics
Yale-New Haven Children's Hospital
New Haven, CT 06510, Connecticut, United States
Presenting Author(s)
Background: Colostrum is regarded as protective against necrotizing enterocolitis and late onset sepsis in very low birth weight ( < 1500 gram) infants when administered to the buccal mucosa as oral immunotherapy (OIT). Additionally, early ( < 6 hours from time of birth) initiation of maternal colostrum expression is associated with a higher probability of providing human milk at time of discharge from the NICU. Our level 3 NICU had neither robust rates of early OIT nor a system in place to ensure initiation of early milk expression.
Objective: To institute a quality improvement (QI) initiative aimed at increasing the percentage of VLBW infants receiving colostrum as OIT within the first 6 hours of life.
Design/Methods: Using the Institute for Healthcare Improvement’s (IHI) Model for Improvement, we implemented interventions aimed at increasing early administration of colostrum to VLBW infants at our level 3 NICU from a baseline mean of 4% to a mean of 50% in a 12-month period (from October 2020 – October 2021). We focused our efforts on early OIT through engaging a multidisciplinary team, including advanced practice providers (APPs), physicians, bedside nurses, lactation consultants (LCs) and parents. We conducted several plan-do-study-act (PDSA) cycles, including updating our evidence-based OIT guidelines, assigning a colostrum champion (CC) for every VLBW admission to obtain and administer colostrum within the first 6 hours of life, optimizing our electronic health record (EHR) use through prompts and alerts, and promoting early LC involvement. Our outcome measure was early OIT administration, which was tracked monthly utilizing control charts. Our process measure included number of breastfeeding (BF) occurrences in the 2 weeks prior to discharge and was tracked monthly using a run chart.
Results: OIT administration increased from a baseline mean of 4% to 64% in the 12-month study period (see figure 1). Our targeted interventions were effective in improving early OIT, and downstream effects in overall administration of OIT to infants of all birth weights were also noted. Our process measure of BF occurrences for VLBW infants in the 2 weeks prior to discharge did not show a significant shift in our median during the study period (see figure 2). Conclusion(s): A multidisciplinary QI initiative led to significant improvement in OIT administration to VLBW infants at a level 3 NICU. The introduction of a CC yielded powerful and lasting change in achieving early colostrum administration. We hope that overtime this will translate to more BF occurrences and human milk at discharge for VLBW infants.
Figure 1. Percentage of VLBW Infants Receiving Early Colostrum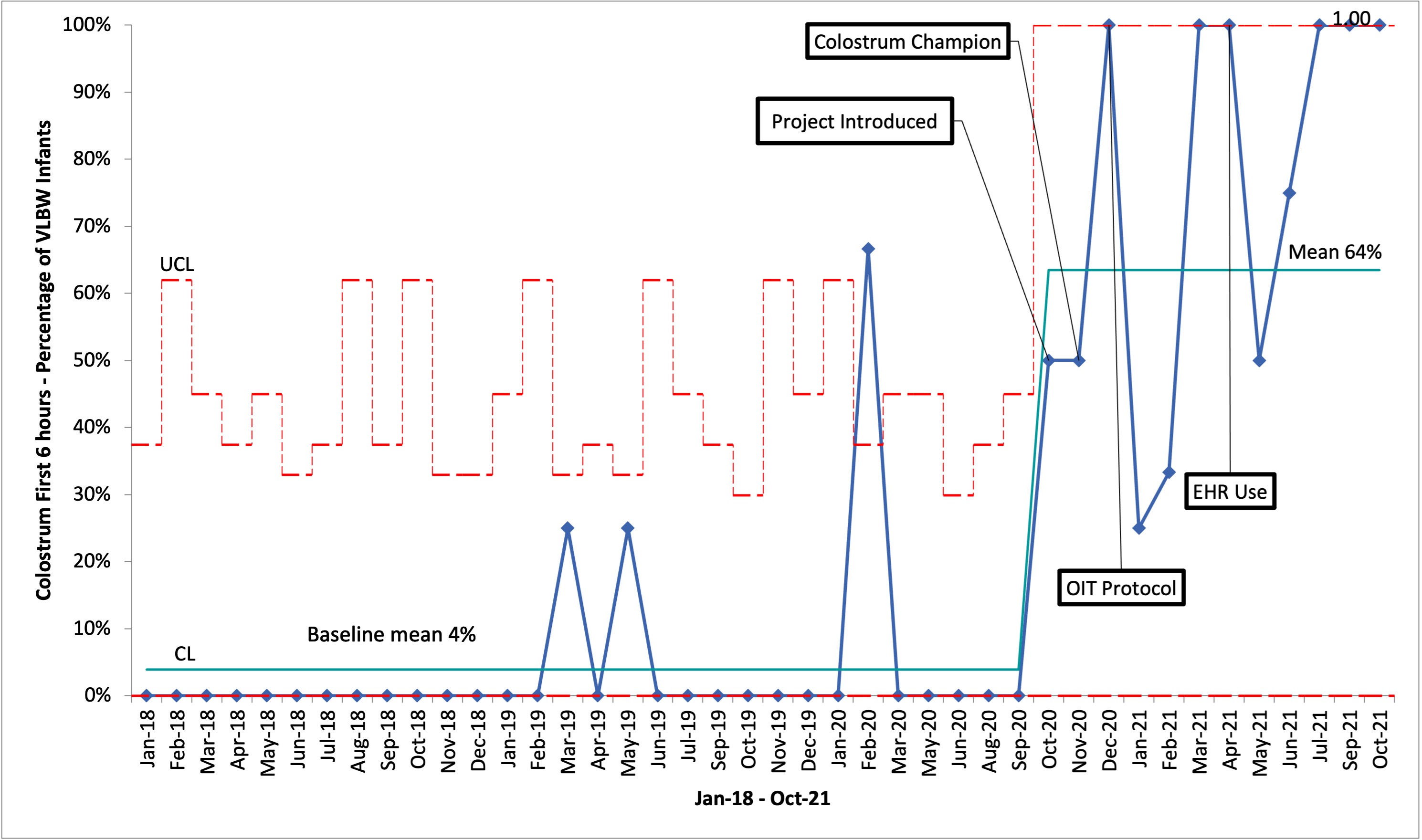 Control chart depicting an increase in our percentage of VLBW infants receiving colostrum as OIT, within the first 6 hours of life, from a baseline of 4% to 64% during our QI initiative.
Control chart depicting an increase in our percentage of VLBW infants receiving colostrum as OIT, within the first 6 hours of life, from a baseline of 4% to 64% during our QI initiative.
Figure 2. Breastfeeding Occurrences for VLBW Infants Prior to Discharge.jpg) Run chart illustrating the median number of BF occurrences for VLBW infants in the two weeks prior to discharge.
Run chart illustrating the median number of BF occurrences for VLBW infants in the two weeks prior to discharge.
Objective: To institute a quality improvement (QI) initiative aimed at increasing the percentage of VLBW infants receiving colostrum as OIT within the first 6 hours of life.
Design/Methods: Using the Institute for Healthcare Improvement’s (IHI) Model for Improvement, we implemented interventions aimed at increasing early administration of colostrum to VLBW infants at our level 3 NICU from a baseline mean of 4% to a mean of 50% in a 12-month period (from October 2020 – October 2021). We focused our efforts on early OIT through engaging a multidisciplinary team, including advanced practice providers (APPs), physicians, bedside nurses, lactation consultants (LCs) and parents. We conducted several plan-do-study-act (PDSA) cycles, including updating our evidence-based OIT guidelines, assigning a colostrum champion (CC) for every VLBW admission to obtain and administer colostrum within the first 6 hours of life, optimizing our electronic health record (EHR) use through prompts and alerts, and promoting early LC involvement. Our outcome measure was early OIT administration, which was tracked monthly utilizing control charts. Our process measure included number of breastfeeding (BF) occurrences in the 2 weeks prior to discharge and was tracked monthly using a run chart.
Results: OIT administration increased from a baseline mean of 4% to 64% in the 12-month study period (see figure 1). Our targeted interventions were effective in improving early OIT, and downstream effects in overall administration of OIT to infants of all birth weights were also noted. Our process measure of BF occurrences for VLBW infants in the 2 weeks prior to discharge did not show a significant shift in our median during the study period (see figure 2). Conclusion(s): A multidisciplinary QI initiative led to significant improvement in OIT administration to VLBW infants at a level 3 NICU. The introduction of a CC yielded powerful and lasting change in achieving early colostrum administration. We hope that overtime this will translate to more BF occurrences and human milk at discharge for VLBW infants.
Figure 1. Percentage of VLBW Infants Receiving Early Colostrum
 Control chart depicting an increase in our percentage of VLBW infants receiving colostrum as OIT, within the first 6 hours of life, from a baseline of 4% to 64% during our QI initiative.
Control chart depicting an increase in our percentage of VLBW infants receiving colostrum as OIT, within the first 6 hours of life, from a baseline of 4% to 64% during our QI initiative.Figure 2. Breastfeeding Occurrences for VLBW Infants Prior to Discharge
.jpg) Run chart illustrating the median number of BF occurrences for VLBW infants in the two weeks prior to discharge.
Run chart illustrating the median number of BF occurrences for VLBW infants in the two weeks prior to discharge.