Health Services Research
Category: Abstract Submission
Health Services Research III
12 - Publication of NIH-Funded Pediatric Clinical Trials
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 12
Publication Number: 12.414
Publication Number: 12.414
Chris A. Rees, Emory University School of Medicine, Atlanta, GA, United States; Claire K. Narang, Boston Children's Hospital, Boston, MA, United States; Adrianna Westbrook, Emory, Atlanta, GA, United States; Florence Bourgeois, Boston Children's Hospital, Boston, MA, United States

Chris A. Rees, MD, MPH (he/him/his)
Assistant Professor
Emory University School of Medicine
ATLANTA, Georgia, United States
Presenting Author(s)
Background: The National Institutes of Health (NIH) is the largest source of government funding for biomedical research in the world, though only a small proportion of its funding is dedicated to pediatric research. Timely dissemination of results of NIH-funded pediatric clinical trials is imperative to maximize scientific impact and ensure the greatest benefit to pediatric clinical care.
Objective: To determine rates of publication of NIH-funded pediatric clinical trials, time to publication, and factors associated with nonpublication.
Design/Methods: We conducted a cross-sectional analysis of NIH grants funding pediatric clinical trials with funding completed in 2017. Our primary outcome was publication of NIH-funded pediatric clinical trials. We identified all grants funding pediatric trials in NIH RePORTER and searched PubMed for publications reporting the results of trials as of December 31, 2021, allowing a minimum of 48 months between funding completion and publication. We assessed time to publication using Kaplan-Meier analysis. We determined factors associated with nonpublication based on an a priori set of variables using multivariable logistic regression.
Results: There were 752 pediatric grants that completed in 2017, of which 143 (19.0%) supported a clinical trial (Table). Substance use (n=20, 14%), mental health conditions (n=19, 13%), and obesity (n=18, 12%) were the most commonly studied conditions. Interventions studied consisted of behavioral interventions in 119 (83%) trials. Twenty-three percent (95% CI 17-31) of trials were published by 24 months and 56% (95% CI 48-64) were published by 48 months after funding completion (Figure). Median time from grant completion to publication was 27.6 months. In adjusted analyses, trials were less likely to remain unpublished if they had larger sample sizes of 100-499 (aOR 0.34, 95% CI 0.12-0.99) or >500 (aOR 0.24, 95% CI 0.06-0.90) participants compared to trials with < 100 participants. Conclusion(s): Almost half of NIH-funded pediatric trials failed to result in published findings 4 years after funding completion. Smaller trials were likely to remain unpublished. These findings may inform future efforts to ensure timely and complete reporting of NIH-funded pediatric trials.
Table. Publication of NIH-Funded Pediatric Clinical Trials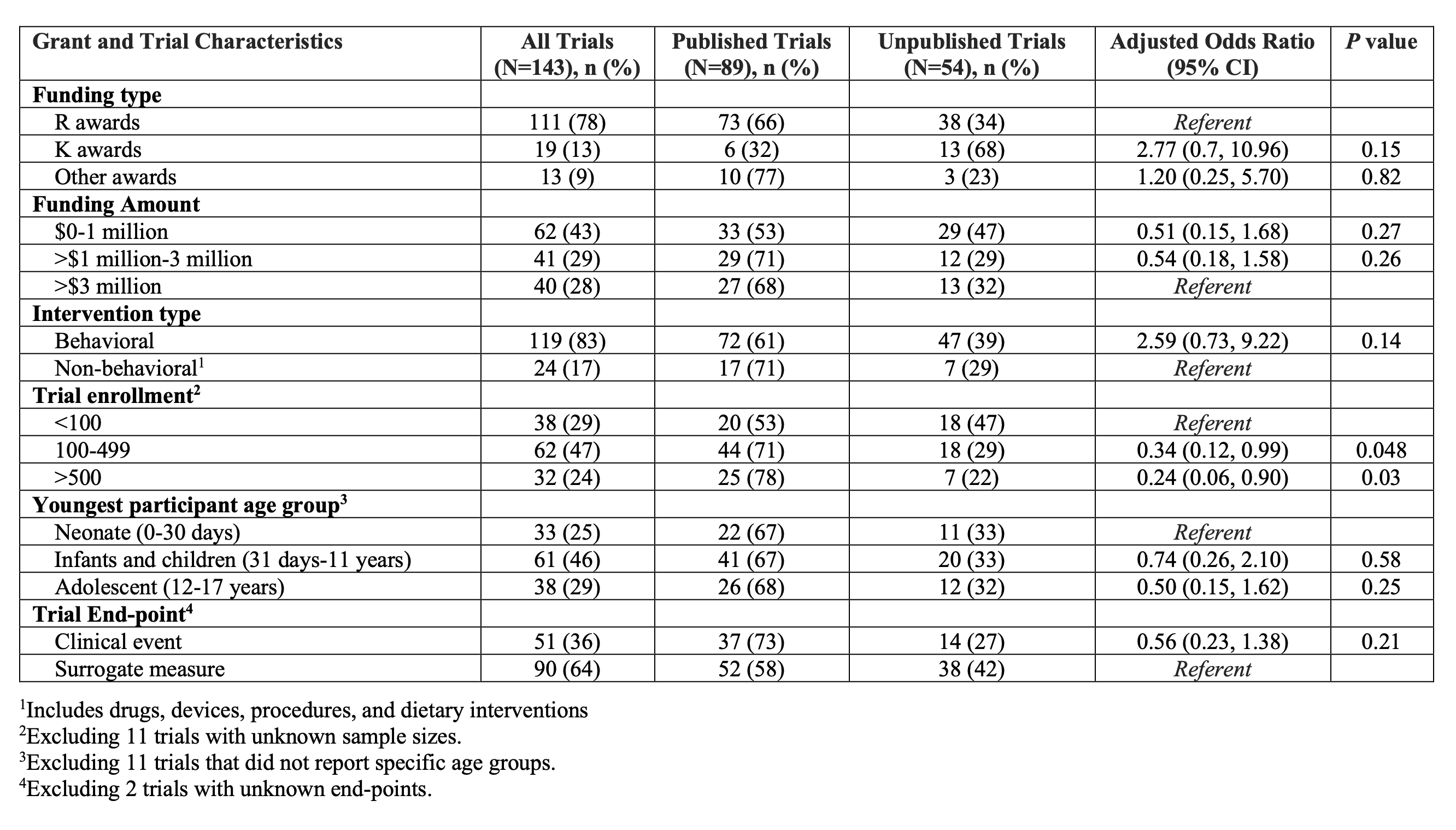
Figure. Kaplan-Meier curve of cumulative incidence of publication of NIH-funded pediatric clinical trials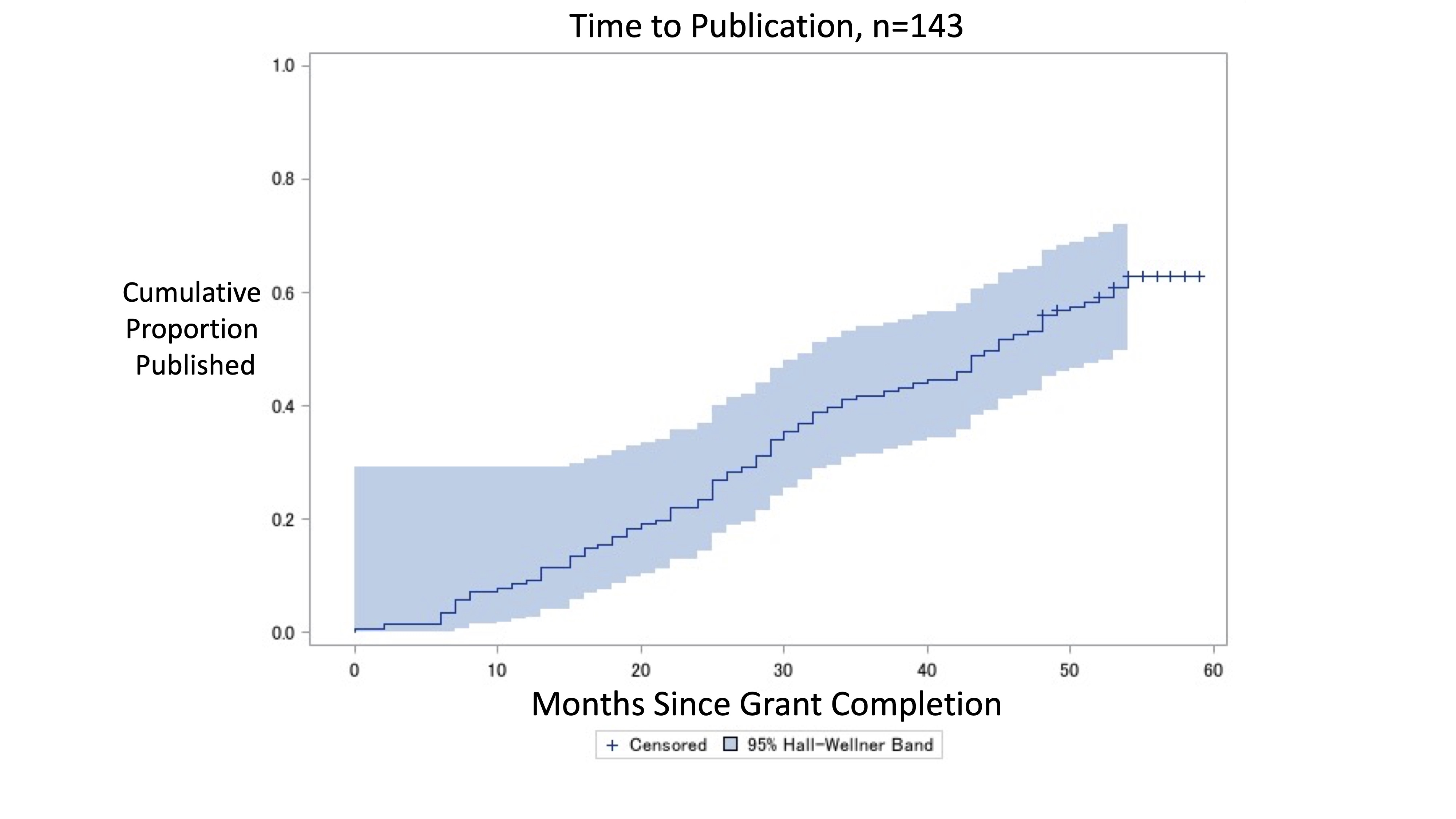
Objective: To determine rates of publication of NIH-funded pediatric clinical trials, time to publication, and factors associated with nonpublication.
Design/Methods: We conducted a cross-sectional analysis of NIH grants funding pediatric clinical trials with funding completed in 2017. Our primary outcome was publication of NIH-funded pediatric clinical trials. We identified all grants funding pediatric trials in NIH RePORTER and searched PubMed for publications reporting the results of trials as of December 31, 2021, allowing a minimum of 48 months between funding completion and publication. We assessed time to publication using Kaplan-Meier analysis. We determined factors associated with nonpublication based on an a priori set of variables using multivariable logistic regression.
Results: There were 752 pediatric grants that completed in 2017, of which 143 (19.0%) supported a clinical trial (Table). Substance use (n=20, 14%), mental health conditions (n=19, 13%), and obesity (n=18, 12%) were the most commonly studied conditions. Interventions studied consisted of behavioral interventions in 119 (83%) trials. Twenty-three percent (95% CI 17-31) of trials were published by 24 months and 56% (95% CI 48-64) were published by 48 months after funding completion (Figure). Median time from grant completion to publication was 27.6 months. In adjusted analyses, trials were less likely to remain unpublished if they had larger sample sizes of 100-499 (aOR 0.34, 95% CI 0.12-0.99) or >500 (aOR 0.24, 95% CI 0.06-0.90) participants compared to trials with < 100 participants. Conclusion(s): Almost half of NIH-funded pediatric trials failed to result in published findings 4 years after funding completion. Smaller trials were likely to remain unpublished. These findings may inform future efforts to ensure timely and complete reporting of NIH-funded pediatric trials.
Table. Publication of NIH-Funded Pediatric Clinical Trials

Figure. Kaplan-Meier curve of cumulative incidence of publication of NIH-funded pediatric clinical trials

