Infectious Diseases
Category: Abstract Submission
Infectious Diseases: COVID-19
297 - Feasibility of At-Home Virological and Serological Testing for SARS-CoV-2 in Children
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 297
Publication Number: 297.215
Publication Number: 297.215
Amina Ahmed, Levine Children's Hospital, Charlotte, NC, United States; Lauren C. Lu, Atrium Health, Charlotte, NC, United States; Casey Stephens, Atrium Health, Charlotte, NC, United States; Anna Harris, Atrium Health, Charlotte, NC, United States; Whitney Rossman, Atrium Health, Charlotte, NC, United States; Keerti Dantuluri, Levine Children's Hospital, Charlotte, NC, United States

Keerti Dantuluri, MD, MPH
Assistant Professor of Pediatric Infectious Diseases
Levine Children's Hospital at Atrium Health
Charlotte, North Carolina, United States
Presenting Author(s)
Background: As the COVID-19 pandemic evolves, longitudinal virological and serological surveillance is critical to inform public health measures. Although viral testing detects acute infection, serosurveillance is needed to capture missed infections, particularly in children, a substantial proportion of whom are asymptomatic. Seroprevalence studies in children are limited by use of residual serum samples. Increased test capacity for longitudinal data will require at-home testing, but the uptake of this in children is unknown. We describe the feasibility of serial at-home virological and serological testing in children participating in a syndromic surveillance study.
Objective: To demonstrate the feasibility of serial at-home virological and serological testing for SARS-CoV-2 in children.
Design/Methods: The COVID-19 Community Research Partnership is a multi-site study of electronic symptom surveillance and serological/virological surveillance. This analysis is for children 2-17 years enrolled at Atrium Health, serving North and South Carolina. Testing was done monthly by participants or parents/guardians. We shipped kits for saliva and fingerprick blood collection; virology specimens were mailed back for PCR testing. A smartphone app was used with an IgM/IgG nucleocapsid antibody test to upload test results. Descriptive statistics examined vaccination status and IgG/IgM test results for patients stratified by age.
Results: Of 1549 children enrolled April 2-June 24, 2021, 1453 (93.8%) agreed to at-home testing; 916 (63.0%) returned at least 1 virology specimen and 623 (42.9%) uploaded at least 1 antibody test result (Table 1).
Only 4 (0.4%) children submitting virology specimens tested positive by PCR during the study. Overall, 76 (35%) of unvaccinated children had a positive IgG. Of unvaccinated children 2-4 years, 13(19.1%) were seropositive; of those 5-11 years, 25(26.9%) were seropositive. Of children ≥ 12 years, 211 (77.8%) were vaccinated and 196 (92.9%) were seropositive; of unvaccinated, 38(63.3%) were seropositive.Conclusion(s): We demonstrate the feasibility of at-home virological and serological testing in children. Uptake of virology testing was higher than with antibody testing. However, even younger children participated in antibody testing. Among those completing antibody testing, the proportion submitting ≥ 4 tests was similar to those submitting 1 test, demonstrating adherence (Figure 1). At-home virological and serological testing supports longitudinal assessment of SARS CoV-2 transmission in children to better inform mitigation strategies in households, daycare centers and schools.
Table 1. Demographics of children who completed one or more serology tests and virology tests..jpg)
Figure 1. Number of serology tests uploaded by participants over time.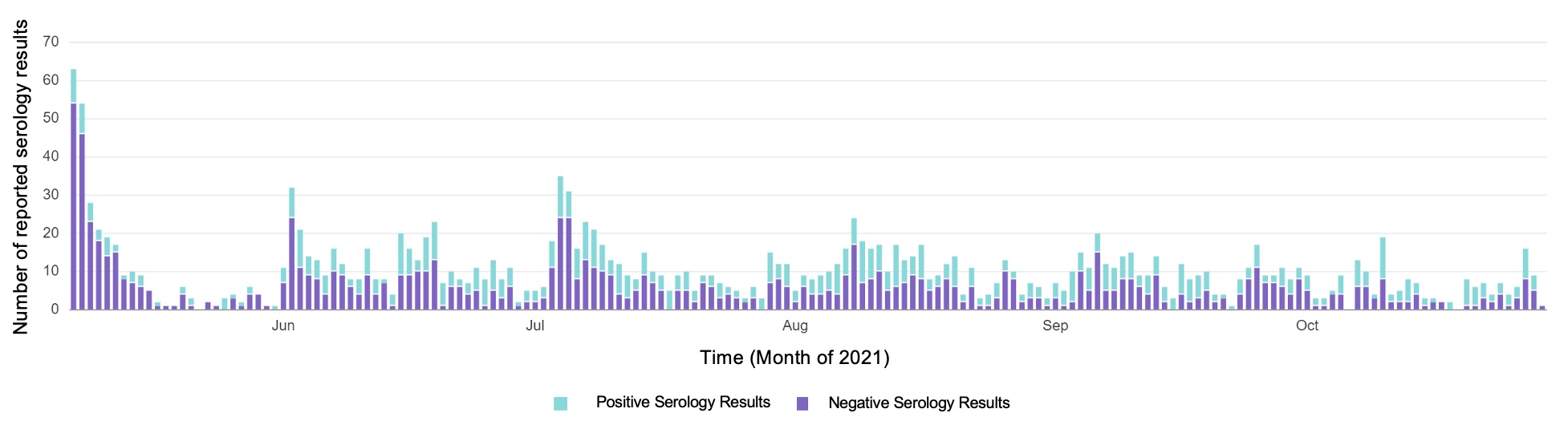
Objective: To demonstrate the feasibility of serial at-home virological and serological testing for SARS-CoV-2 in children.
Design/Methods: The COVID-19 Community Research Partnership is a multi-site study of electronic symptom surveillance and serological/virological surveillance. This analysis is for children 2-17 years enrolled at Atrium Health, serving North and South Carolina. Testing was done monthly by participants or parents/guardians. We shipped kits for saliva and fingerprick blood collection; virology specimens were mailed back for PCR testing. A smartphone app was used with an IgM/IgG nucleocapsid antibody test to upload test results. Descriptive statistics examined vaccination status and IgG/IgM test results for patients stratified by age.
Results: Of 1549 children enrolled April 2-June 24, 2021, 1453 (93.8%) agreed to at-home testing; 916 (63.0%) returned at least 1 virology specimen and 623 (42.9%) uploaded at least 1 antibody test result (Table 1).
Only 4 (0.4%) children submitting virology specimens tested positive by PCR during the study. Overall, 76 (35%) of unvaccinated children had a positive IgG. Of unvaccinated children 2-4 years, 13(19.1%) were seropositive; of those 5-11 years, 25(26.9%) were seropositive. Of children ≥ 12 years, 211 (77.8%) were vaccinated and 196 (92.9%) were seropositive; of unvaccinated, 38(63.3%) were seropositive.Conclusion(s): We demonstrate the feasibility of at-home virological and serological testing in children. Uptake of virology testing was higher than with antibody testing. However, even younger children participated in antibody testing. Among those completing antibody testing, the proportion submitting ≥ 4 tests was similar to those submitting 1 test, demonstrating adherence (Figure 1). At-home virological and serological testing supports longitudinal assessment of SARS CoV-2 transmission in children to better inform mitigation strategies in households, daycare centers and schools.
Table 1. Demographics of children who completed one or more serology tests and virology tests.
.jpg)
Figure 1. Number of serology tests uploaded by participants over time.

