Neonatal General
Category: Abstract Submission
Neonatology General 3
378 - Neonatal Extracorporeal Life Support: Associations between Continuous Renal Replacement Therapy, Thrombocytopenia, and Outcomes
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 378
Publication Number: 378.133
Publication Number: 378.133
Lauren R. Walker, Medical University of South Carolina College of Medicine, Charleston, SC, United States; W. Michael Southgate, Medical University of South Carolina College of Medicine, Edisto Island, SC, United States; David Selewski, Medical University of South Carolina College of Medicine, Charleston, SC, United States; Laura E. Hollinger, Medical University of South Carolina College of Medicine, Charleston, SC, United States; Jeffrey E. Korte, Medical University of South Carolina, Charleston, SC, United States; Heidi J. Steflik, Medical University of South Carolina, Charleston, SC, United States

Lauren R. Walker, MD (she/her/hers)
Neonatal Perinatal Fellow
Medical University of South Carolina College of Medicine
Charleston, South Carolina, United States
Presenting Author(s)
Background: Concurrent use of continuous renal replacement therapy (CRRT) during extracorporeal life support (ECLS) is common, but under-studied in neonates. Thrombocytopenia (TP) has been associated with CRRT receipt in adults, however the incidence of TP in neonates receiving ECLS with concurrent CRRT and associated complications have not been described.
Objective: We hypothesized that CRRT receipt during neonatal ECLS would be associated with increased ECLS complications, prolonged length of stay (LOS), and mortality. Secondarily, we hypothesized that CRRT would be associated with severe TP, and those with severe TP would experience more ECLS complications, prolonged LOS, and increased mortality.
Design/Methods: We conducted a chart review of neonates who received ECLS at our institution 07/01/14 - 03/01/20. CRRT was prescribed at the discretion of the provider in the pediatric or cardiac intensive care unit (ICU); all patients in the neonatal ICU received CRRT. We compared baseline characteristics, ECLS complications, LOS, and mortality between those who did and did not receive concurrent CRRT. We assessed for association between CRRT receipt and severe TP (platelets < 50,000). Among CRRT receivers, we conducted similar comparisons between those with minimum platelets ≥50,000 (mild TP) and < 50,000 (severe TP).
Results: Fifty-two neonates were included; 35 neonates received CRRT (Figure). Diagnosis prompting ECLS (p < 0.01) and ECLS mode (p=0.01) differed between those who did and did not receive concurrent CRRT (Table 1). Those who received concurrent CRRT experienced more moderate or severe hemolysis (p=0.03) than non-CRRT receivers, while other ECLS complications, LOS, and mortality did not differ between groups (Table 2). There was no association between CRRT receipt and severe TP.
Among CRRT receivers, 21 neonates developed severe TP. Compared to CRRT receivers with mild TP, those with severe TP had lower birth weight (p=0.04) and increased incidence of confirmed infection (severe TP: 6/21 (29%) vs. mild TP: 0 (0%); p=0.03). There were no differences in CRRT modality, ECLS complications, LOS, or mortality between groups.Conclusion(s): In our cohort, patients who received ECLS with concurrent CRRT experienced more hemolysis than those who did not receive CRRT, but CRRT receipt was not associated with severe TP, prolonged LOS, or increased mortality. In CRRT receivers, there was an increased incidence of confirmed infection in those with severe TP, but no differences in ECLS complications, LOS, or mortality compared to those with mild TP.
Figure- Study Enrollment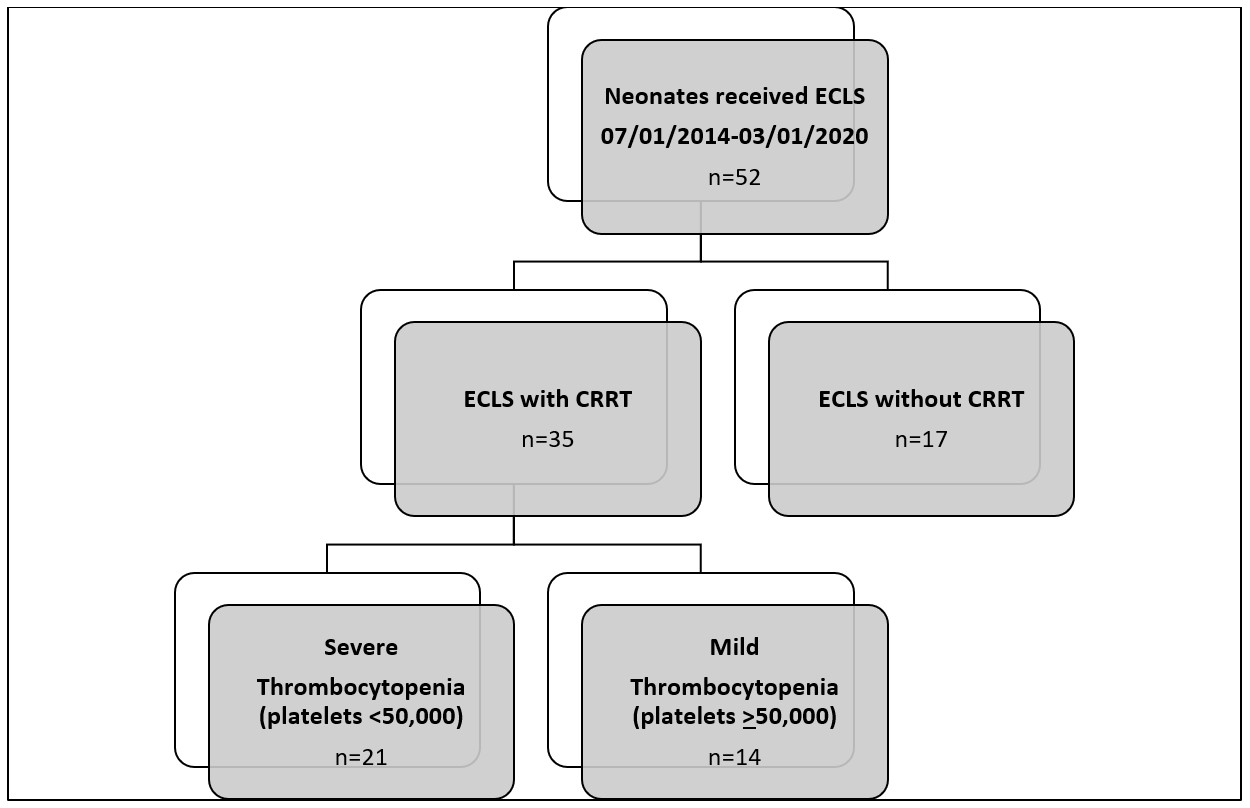
Table 1 – Demographics and Baseline Characteristics of Neonates Receiving Extracorporeal Life Support with and without Concurrent Continuous Renal Replacement Therapy
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/16020/FGOVBGGC-1174543-2-IMG.jpg width=440 hheight=337.987751531059 border=0 style=border-style: none;>Continuous variables displayed as mean ± standard deviation; categorical variables displayed as count (percentage). ECLS, extracorporeal life support; CRRT, continuous renal replacement therapy; CDH, congenital diaphragmatic hernia; VA, venoarterial; VV, venovenous; CVVH, continuous venovenous hemofiltration; CVVHDF, continuous venovenous hemodiafiltration.
a: p-value representative of comparisons between ECLS only (no CRRT) and ECLS with CRRT receivers.
b: p-value representative of comparisons between ECLS with CRRT receivers with minimum platelet count < 50,000 and ECLS with CRRT receivers with minimum platelet count >50,000.
Objective: We hypothesized that CRRT receipt during neonatal ECLS would be associated with increased ECLS complications, prolonged length of stay (LOS), and mortality. Secondarily, we hypothesized that CRRT would be associated with severe TP, and those with severe TP would experience more ECLS complications, prolonged LOS, and increased mortality.
Design/Methods: We conducted a chart review of neonates who received ECLS at our institution 07/01/14 - 03/01/20. CRRT was prescribed at the discretion of the provider in the pediatric or cardiac intensive care unit (ICU); all patients in the neonatal ICU received CRRT. We compared baseline characteristics, ECLS complications, LOS, and mortality between those who did and did not receive concurrent CRRT. We assessed for association between CRRT receipt and severe TP (platelets < 50,000). Among CRRT receivers, we conducted similar comparisons between those with minimum platelets ≥50,000 (mild TP) and < 50,000 (severe TP).
Results: Fifty-two neonates were included; 35 neonates received CRRT (Figure). Diagnosis prompting ECLS (p < 0.01) and ECLS mode (p=0.01) differed between those who did and did not receive concurrent CRRT (Table 1). Those who received concurrent CRRT experienced more moderate or severe hemolysis (p=0.03) than non-CRRT receivers, while other ECLS complications, LOS, and mortality did not differ between groups (Table 2). There was no association between CRRT receipt and severe TP.
Among CRRT receivers, 21 neonates developed severe TP. Compared to CRRT receivers with mild TP, those with severe TP had lower birth weight (p=0.04) and increased incidence of confirmed infection (severe TP: 6/21 (29%) vs. mild TP: 0 (0%); p=0.03). There were no differences in CRRT modality, ECLS complications, LOS, or mortality between groups.Conclusion(s): In our cohort, patients who received ECLS with concurrent CRRT experienced more hemolysis than those who did not receive CRRT, but CRRT receipt was not associated with severe TP, prolonged LOS, or increased mortality. In CRRT receivers, there was an increased incidence of confirmed infection in those with severe TP, but no differences in ECLS complications, LOS, or mortality compared to those with mild TP.
Figure- Study Enrollment

Table 1 – Demographics and Baseline Characteristics of Neonates Receiving Extracorporeal Life Support with and without Concurrent Continuous Renal Replacement Therapy
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/16020/FGOVBGGC-1174543-2-IMG.jpg width=440 hheight=337.987751531059 border=0 style=border-style: none;>Continuous variables displayed as mean ± standard deviation; categorical variables displayed as count (percentage). ECLS, extracorporeal life support; CRRT, continuous renal replacement therapy; CDH, congenital diaphragmatic hernia; VA, venoarterial; VV, venovenous; CVVH, continuous venovenous hemofiltration; CVVHDF, continuous venovenous hemodiafiltration.
a: p-value representative of comparisons between ECLS only (no CRRT) and ECLS with CRRT receivers.
b: p-value representative of comparisons between ECLS with CRRT receivers with minimum platelet count < 50,000 and ECLS with CRRT receivers with minimum platelet count >50,000.
