Neonatal Fetal Nutrition & Metabolism
Category: Abstract Submission
Neonatal Fetal Nutrition & Metabolism I
271 - A Randomized Controlled Trial (RCT) Investigating the Impact of L-carnitine Supplementation on NICU Network Neurobehavioral Scale Scores in Preterm Infants
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 271
Publication Number: 271.119
Publication Number: 271.119
Marie A. Clark, Case Western Reserve University School of Medicine, Cleveland, OH, United States; Indirapriya Darshini Avulakunta, Childrens hospital at Montefiore, Bronx, NY, United States; Ellen J. Silver, Albert Einstein College of Medicine, Mount Kisco, NY, United States; Ruth EK Stein, Albert Einstein College of Medicine, Bronx, NY, United States; Nora Esteban-Cruciani, Albert Einstein Medical Center, Jefferson University, Philadelphia, PA, United States; Mamta Fuloria, The Children's Hospital at Montefiore, Bronx, NY, United States
- IA
Indirapriya Darshini Avulakunta, MD
Neonatal Perinatal Medicine Fellow
Childrens hospital at Montefiore
Bronx, New York, United States
Presenting Author(s)
Background: Carnitine facilitates beta oxidation of mitochondrial fatty acids and demonstrates neuroprotective effects by decreasing oxidative stress and preventing subsequent cell death in animal models of neonatal brain injury. It is unknown if carnitine supplementation improves NICU Network Neurobehavioral Scale (NNNS) scores in preterm infants born at < 32 weeks gestation.
Objective: To examine the effect of early supplementation of L-carnitine on NNNS scores in preterm infants born at < 32 weeks gestation. We hypothesized that preterm infants supplemented early with physiologic doses of L-carnitine would demonstrate a more favorable pattern of NNNS subscale scores compared to control infants.
Design/Methods: We conducted a prospective, double-blind RCT with infants born at < 32 weeks gestation admitted to the Neonatal Intensive Care Unit (NICU) and randomized to receive 50 μmol/kg/day of L-carnitine or an equivalent volume of 5% dextrose for a minimum of 2 weeks or until enteral intake of 100 ml/kg/day was achieved. NNNS exams were conducted by trained providers at 38 weeks’ post-menstrual age +/- 1 week. Differences between groups were examined using the Mann Whitney U test. A p-value < 0.05 was considered statistically significant.
Results: 144 infants were enrolled, 72 in each study group. One infant from carnitine and three from placebo group withdrew from the study. Four infants died within the first 2 weeks of life, leaving 136 infants (97.15% survival) in the study. (Table 1). Infants randomized to the carnitine group were more likely to require intubation at delivery (p < 0.01). Mothers of infants in the control group were more likely to have received antenatal magnesium sulfate (p=0.01) and antibiotics (p=0.008) and were more likely to have chorioamnionitis (p=0.041). Of enrolled infants, 119 received a NNNS exam. There were no significant differences between infants who received NNNS testing v. those without. Infants in the carnitine group had a lower NNNS hypotonicity score (p < 0.05). There was no significant difference in other subscale scores (Table 2). Stratifying by gestational age and gender did not significantly impact scores.Conclusion(s): Early supplementation with physiologic doses of L-carnitine was associated with a lower hypotonicity score on the NNNS. Future studies are necessary to determine any potential longer-term impact of carnitine supplementation on neurobehavioral outcomes.
Table 1: Baseline Maternal and Infant Characteristics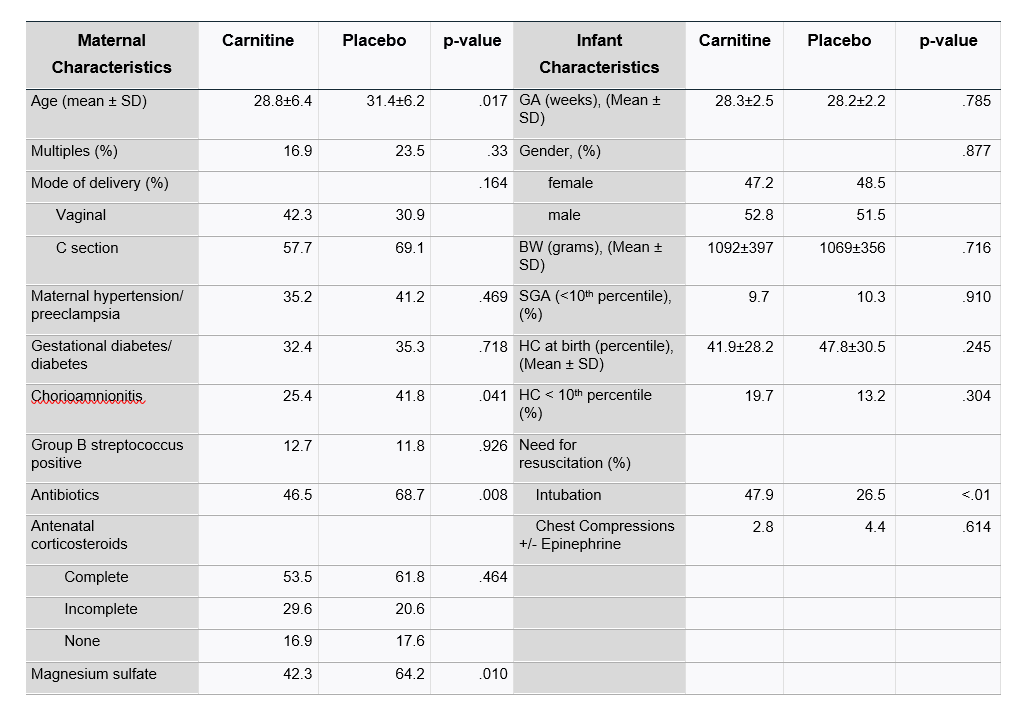
Table 2: NNNS Subscale Scores in Carnitine v. Placebo Groups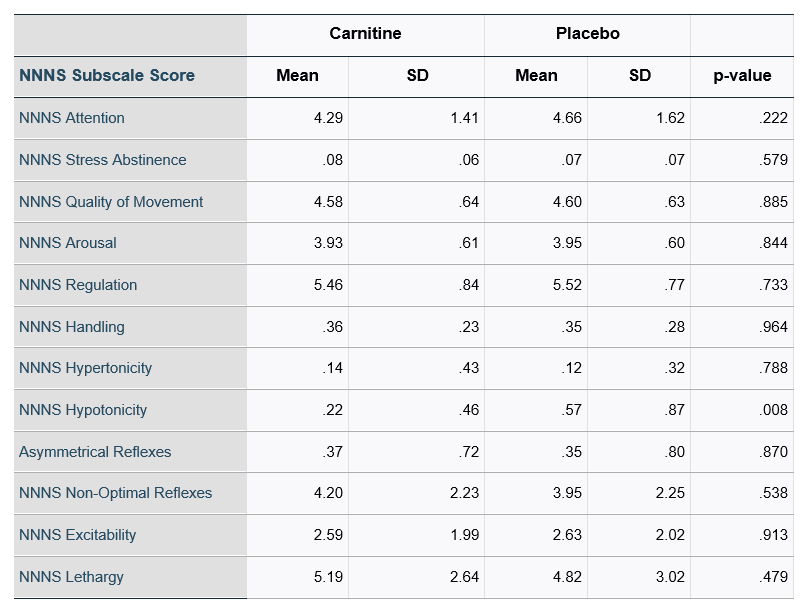
Objective: To examine the effect of early supplementation of L-carnitine on NNNS scores in preterm infants born at < 32 weeks gestation. We hypothesized that preterm infants supplemented early with physiologic doses of L-carnitine would demonstrate a more favorable pattern of NNNS subscale scores compared to control infants.
Design/Methods: We conducted a prospective, double-blind RCT with infants born at < 32 weeks gestation admitted to the Neonatal Intensive Care Unit (NICU) and randomized to receive 50 μmol/kg/day of L-carnitine or an equivalent volume of 5% dextrose for a minimum of 2 weeks or until enteral intake of 100 ml/kg/day was achieved. NNNS exams were conducted by trained providers at 38 weeks’ post-menstrual age +/- 1 week. Differences between groups were examined using the Mann Whitney U test. A p-value < 0.05 was considered statistically significant.
Results: 144 infants were enrolled, 72 in each study group. One infant from carnitine and three from placebo group withdrew from the study. Four infants died within the first 2 weeks of life, leaving 136 infants (97.15% survival) in the study. (Table 1). Infants randomized to the carnitine group were more likely to require intubation at delivery (p < 0.01). Mothers of infants in the control group were more likely to have received antenatal magnesium sulfate (p=0.01) and antibiotics (p=0.008) and were more likely to have chorioamnionitis (p=0.041). Of enrolled infants, 119 received a NNNS exam. There were no significant differences between infants who received NNNS testing v. those without. Infants in the carnitine group had a lower NNNS hypotonicity score (p < 0.05). There was no significant difference in other subscale scores (Table 2). Stratifying by gestational age and gender did not significantly impact scores.Conclusion(s): Early supplementation with physiologic doses of L-carnitine was associated with a lower hypotonicity score on the NNNS. Future studies are necessary to determine any potential longer-term impact of carnitine supplementation on neurobehavioral outcomes.
Table 1: Baseline Maternal and Infant Characteristics

Table 2: NNNS Subscale Scores in Carnitine v. Placebo Groups

