Infectious Diseases
Category: Abstract Submission
Infectious Diseases: Bacteria & Antimicrobials
296 - Clinical Outcome Comparison Between ESBL Versus non ESBL Urinary Tract Infection in Children
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 296
Publication Number: 296.114
Publication Number: 296.114
Xhesika Begaj, New York University Long Island School of Medicine, New York, NY, United States; Hannah Lee, UT Southwestern, Dallas, TX, United States; Asif Noor, The Children’s Medical Center at NYU Winthrop Hospital, Mineola, NY, United States; Theresa Fiorito, The Children’s Medical Center at NYU Winthrop Hospital, Mineola, NY, United States; Vipin Agarwalla, NYU Langone Health- Long Island (WINTHROP) HOSPITAL, Roslyn, NY, United States; Ooha Kambhampati, NYU Langone Long Island, Mineola, NY, United States; Shahidul Islam, New York University Long Island School of Medicine, Mineola, NY, United States; Leonard R. Krilov, The Children’s Medical Center at NYU Winthrop Hospital, Mineola, NY, United States

Xhesika Begaj
Second-Year Medical Student
New York University Long Island School of Medicine
New York, New York, United States
Presenting Author(s)
Background: Extended spectrum beta-lactamase-producing (ESBL) Enterobacteriacea has been increasingly associated with community-acquired urinary tract infections (UTIs) in children worldwide. Most epidemiological studies on ESBL UTIs have been performed outside the U.S. The treatment of choice in ESBL UTI is a carbapenem antibiotic. At our institution, located in New York, all children diagnosed with UTI are started empirically on a third-generation cephalosporin and definitive antibiotic treatment in based on urine culture results.
Objective: The aim of our study was to compare the clinical outcomes in children diagnosed with ESBL UTI and non-ESBL UTI, who received a non carbapenem empiric antibiotic. In addition we studied patient and laboratory characteristics as well as risk factors in non-ESBL and ESBL groups.
Design/Methods: A retrospective analysis was performed on patients diagnosed with UTI, aged 0 days to 19 years. This IRB approved study included patients from April 2014 to July 2018. The primary outcomes were length of hospital stay, fever duration, 30-day readmission rate, and 7-day revisit rate. Results were considered statistically significant at a p value < 0.05 and were analyzed via Wilcoxon rank-sum test, Fisher’s exact test, and multivariable logistic regression.
Results: A total of 627 patients were included in the study, of which the majority were female in both ESBL (80.4%, n = 56) and non-ESBL groups (81.6%, n = 571) with median ages of 1.5 years and 1.9 years, respectively. Patients with ESBL UTIs had a more frequent history of recurrent UTIs (41.1% vs. 19.4%, p < 0.001), recent antibiotic use (32.1% vs. 11.7%, p < 0.001), and recent hospitalization (32.1% vs. 13.0%, p < 0.001). Pyruia and serum leukocytosis between the two groups did not differ significantly. Empiric third-generation cephalosporin antibiotics was administered in 76.8% of ESBL UTIs and 72.2% of the non-ESBL UTIs (Table 1).
The primary outcomes showed no difference in fever duration, in contrast to the significantly longer length of hospital stay, higher 30-day readmission rate, and higher 7-day revisit rate in ESBL versus non-ESBL patients (Table 2).Conclusion(s): This study found no significant difference in fever duration in ESBL versus non-ESBL group in the setting of empiric third-generation cephalosporin. The length of stay, and readmission as well as revisit rate was significantly higher in the ESBL group.
Table 1. Demographics and clinical characteristics of ESBL and non-ESBL groups.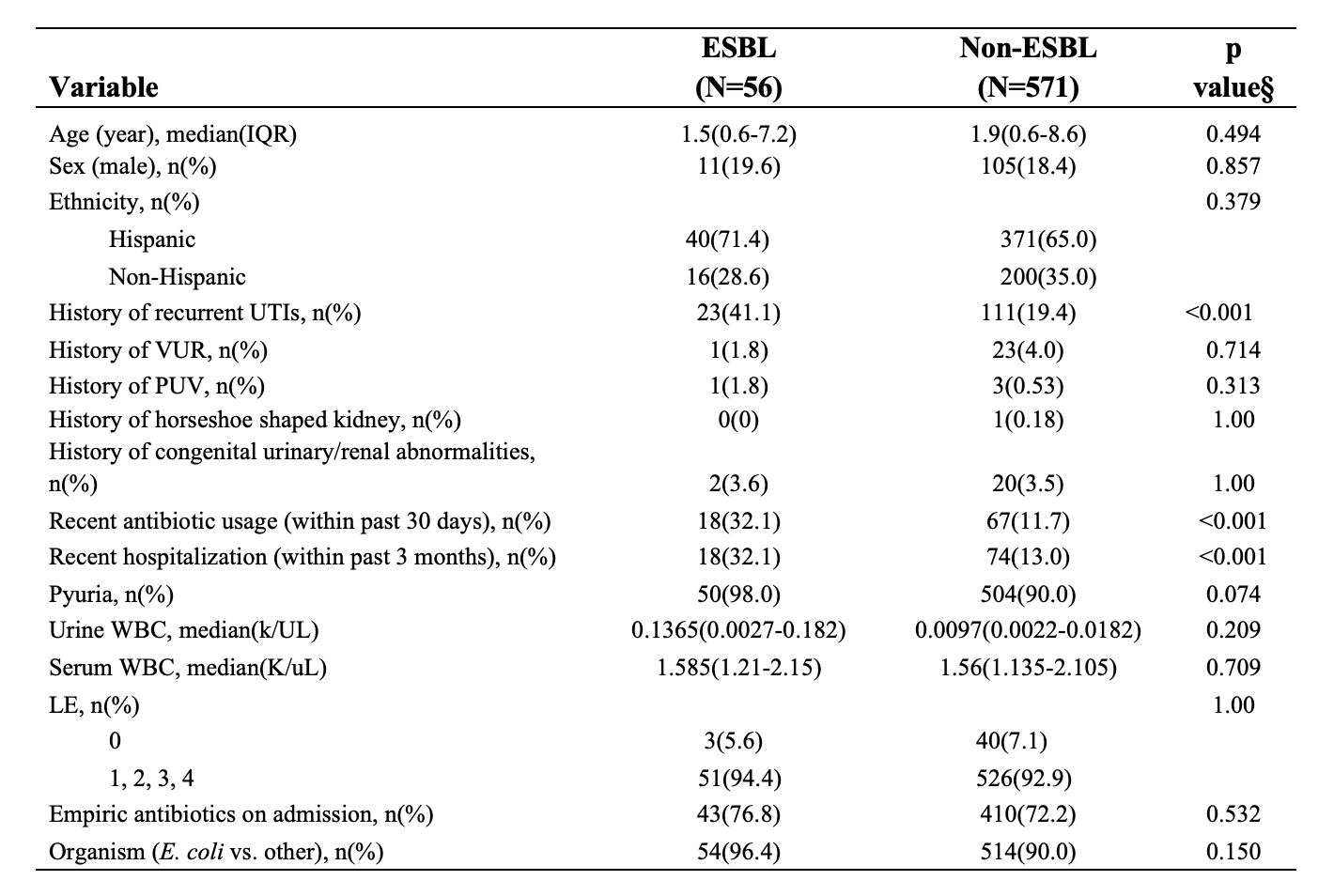 §P-values are from Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
§P-values are from Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
Table 2. Bivariate analysis of primary outcomes between ESBL and non-ESBL UTIs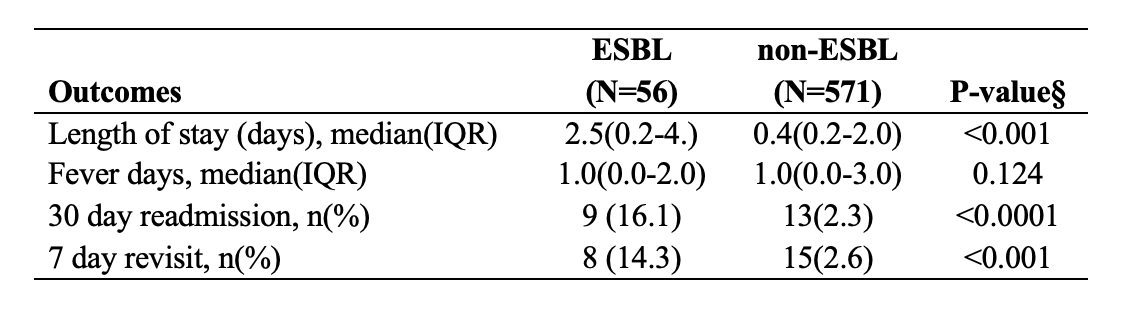 § P values are from Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
§ P values are from Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
Objective: The aim of our study was to compare the clinical outcomes in children diagnosed with ESBL UTI and non-ESBL UTI, who received a non carbapenem empiric antibiotic. In addition we studied patient and laboratory characteristics as well as risk factors in non-ESBL and ESBL groups.
Design/Methods: A retrospective analysis was performed on patients diagnosed with UTI, aged 0 days to 19 years. This IRB approved study included patients from April 2014 to July 2018. The primary outcomes were length of hospital stay, fever duration, 30-day readmission rate, and 7-day revisit rate. Results were considered statistically significant at a p value < 0.05 and were analyzed via Wilcoxon rank-sum test, Fisher’s exact test, and multivariable logistic regression.
Results: A total of 627 patients were included in the study, of which the majority were female in both ESBL (80.4%, n = 56) and non-ESBL groups (81.6%, n = 571) with median ages of 1.5 years and 1.9 years, respectively. Patients with ESBL UTIs had a more frequent history of recurrent UTIs (41.1% vs. 19.4%, p < 0.001), recent antibiotic use (32.1% vs. 11.7%, p < 0.001), and recent hospitalization (32.1% vs. 13.0%, p < 0.001). Pyruia and serum leukocytosis between the two groups did not differ significantly. Empiric third-generation cephalosporin antibiotics was administered in 76.8% of ESBL UTIs and 72.2% of the non-ESBL UTIs (Table 1).
The primary outcomes showed no difference in fever duration, in contrast to the significantly longer length of hospital stay, higher 30-day readmission rate, and higher 7-day revisit rate in ESBL versus non-ESBL patients (Table 2).Conclusion(s): This study found no significant difference in fever duration in ESBL versus non-ESBL group in the setting of empiric third-generation cephalosporin. The length of stay, and readmission as well as revisit rate was significantly higher in the ESBL group.
Table 1. Demographics and clinical characteristics of ESBL and non-ESBL groups.
 §P-values are from Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
§P-values are from Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.Table 2. Bivariate analysis of primary outcomes between ESBL and non-ESBL UTIs
 § P values are from Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.
§ P values are from Wilcoxon rank-sum test for continuous variables and Fisher’s exact test for categorical variables.