Neonatal Fetal Nutrition & Metabolism
Category: Abstract Submission
Neonatal Fetal Nutrition & Metabolism II
286 - Early Total Enteral Feeding in Preterm Infants 26-34 weeks’ Gestation with Respiratory Distress Syndrome on Respiratory Support
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 286
Publication Number: 286.120
Publication Number: 286.120
Sushma Nangia, Lady Hardinge Medical College & Kalawati Saran Children's Hospital, New Delhi, Delhi, India; Rohit Anand, Others, New Delhi, Delhi, India; Gunjana Kumar, others, NEW DELHI, India, Delhi, India

Sushma Nangia, MBBS, MD, DM
Neonatologist
Lady Hardinge Medical College & Kalawati Saran Children's Hospital
New Delhi, Delhi, India
Presenting Author(s)
Background: Providing adequate nutrition in the management of preterm infants has been challenging. Fear of feed intolerance (FI) and necrotizing enterocolitis (NEC) are the two main reasons for delayed initiation and slow advancement of enteral feeds. Feasibility of Early total enteral feeding (ETEF) in stable very low birth weight infants has been documented.
Objective: The objective of this secondary analysis of data from the randomized trial comparing Less invasive surfactant therapy (LISA) with INSURE method of surfactant administration is to demonstrate the feasibility of ETEF in hemodynamically stable preterm neonates on respiratory support and to examine the factors associated with failure of ETEF.
Design/Methods: Randomized controlled trial comparing LISA vs. INSURE among preterm infants between 26-34 weeks of gestation, enrolled 150 infants with 117 being hemodynamically stable. Full volume enteral feeding without any parenteral supplementation was started on day 1 of life using mother’s own milk (MoM) or donor human milk ( <32 weeks GA) and MoM or Preterm formula (33-34 weeks GA) and advanced as per the feeding protocol depicted in table 1. The data was analyzed to assess the proportion of babies developing of feed intolerance and/or NEC and factors associated with failure of ETEF. All Infants were assessed for the day of attainment of full enteral feeding defined as receiving and tolerating 150 ml/kg of enteral feeds per day. Further analysis included proportion of infants developing sepsis, time to regain birth weight, and duration of hospital stay.
Results: Out of these 117 babies, 102 tolerated ETEF and 15 had one or more episodes of FI requiring parenteral supplementation but none developed NEC. On assessment of possible factors associated with ETEF failure, there were no differences in baseline characteristics but statistically significantly increased incidence of culture positive sepsis as well as requirement of antibiotic therapy for possible sepsis in babies with failure of ETEF. The babies who tolerated ETEF achieved full enteral feeding (150 mL/Kg/day) significantly earlier (5.48±1.1 days) compared to those with ETEF failure (7±3.4 days) (p- 0.001). The time to regain birth weight was earlier with a reduction in duration of hospital stay in babies who tolerated ETEF but these results were not statistically significant.Conclusion(s): ETEF is feasible in preterm neonates with respiratory distress syndrome who are on respiratory support. It resulted in earlier attainment of full enteral feeds and decreased the incidence of sepsis with reduced antibiotic usage.
Table1- Enteral feeding protocol (ml/kg) in the study in VLBW and LBW babies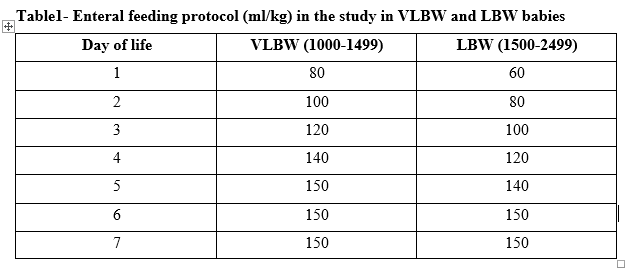
Table2- Baseline characteristics of preterm neonates in study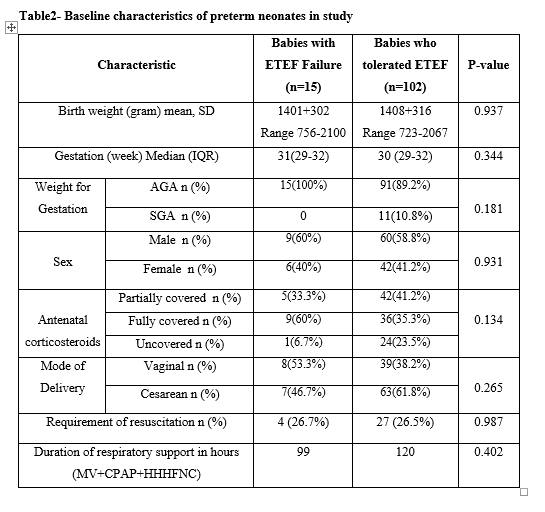
Objective: The objective of this secondary analysis of data from the randomized trial comparing Less invasive surfactant therapy (LISA) with INSURE method of surfactant administration is to demonstrate the feasibility of ETEF in hemodynamically stable preterm neonates on respiratory support and to examine the factors associated with failure of ETEF.
Design/Methods: Randomized controlled trial comparing LISA vs. INSURE among preterm infants between 26-34 weeks of gestation, enrolled 150 infants with 117 being hemodynamically stable. Full volume enteral feeding without any parenteral supplementation was started on day 1 of life using mother’s own milk (MoM) or donor human milk ( <32 weeks GA) and MoM or Preterm formula (33-34 weeks GA) and advanced as per the feeding protocol depicted in table 1. The data was analyzed to assess the proportion of babies developing of feed intolerance and/or NEC and factors associated with failure of ETEF. All Infants were assessed for the day of attainment of full enteral feeding defined as receiving and tolerating 150 ml/kg of enteral feeds per day. Further analysis included proportion of infants developing sepsis, time to regain birth weight, and duration of hospital stay.
Results: Out of these 117 babies, 102 tolerated ETEF and 15 had one or more episodes of FI requiring parenteral supplementation but none developed NEC. On assessment of possible factors associated with ETEF failure, there were no differences in baseline characteristics but statistically significantly increased incidence of culture positive sepsis as well as requirement of antibiotic therapy for possible sepsis in babies with failure of ETEF. The babies who tolerated ETEF achieved full enteral feeding (150 mL/Kg/day) significantly earlier (5.48±1.1 days) compared to those with ETEF failure (7±3.4 days) (p- 0.001). The time to regain birth weight was earlier with a reduction in duration of hospital stay in babies who tolerated ETEF but these results were not statistically significant.Conclusion(s): ETEF is feasible in preterm neonates with respiratory distress syndrome who are on respiratory support. It resulted in earlier attainment of full enteral feeds and decreased the incidence of sepsis with reduced antibiotic usage.
Table1- Enteral feeding protocol (ml/kg) in the study in VLBW and LBW babies

Table2- Baseline characteristics of preterm neonates in study

