Infectious Diseases
Category: Abstract Submission
Infectious Diseases: Bacteria & Antimicrobials
297 - Effect of Suppressing Clostridioides difficile Test Result from Multiplex Gastrointestinal Pathogen Panels
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 297
Publication Number: 297.114
Publication Number: 297.114
Priya K. Patel, All Children’s Hospital Foundation grant, St. Petersburg, FL, United States; Jamie L. Fierstein, Johns Hopkins All Children's Hospital, St. Petersburg, FL, United States; Robert A. Dudas, Johns Hopkins All Children's Hospital, St. Petersburg, FL, United States; John M. Morrison, Johns Hopkins All Children's Hospital, Saint Petersburg, FL, United States
- PP
Priya K. Patel, MD
All Children’s Hospital Foundation grant
St. Petersburg, Florida, United States
Presenting Author(s)
Background: A positive result for C. difficile on a gastrointestinal pathogen panel (GIP) is not definitive for acute C. difficle infection (CDI). In 2016, our institution suppressed C. difficile result when implementing the GIP into practice to prevent inappropriate testing and/or treatment for individuals colonized with C. difficile. Clinicians could order standalone C. difficile nucleic acid amplification test (NAAT) if they clinically suspected CDI. The effect of C. difficile result suppression on provider ordering behaviors and clinical outcomes will provide insight into diagnostic stewardship for C. difficile testing.
Objective: Among patients with positive C. difficile testing (NAAT +/- GIP), we aimed to determine if the presence of CDI diagnostic criteria and risk factors are associated with 1) obtaining targeted C. difficile NAAT testing or 2) coinfection with C. difficile and other pathogen and 3) if time to diagnosis and time to treatment differed between patients with CDI receiving both NAAT and GIP compared to those receiving NAAT testing alone.
Design/Methods: We performed a retrospective single center study from 2/2/2016 to 12/17/2018 of inpatient and outpatients with positive C. difficile test result (NAAT or GIP, Fig. 1). The primary study exposure was obtaining targeted C. difficile testing (NAAT +/- GIP; visible) or not (GIP alone; suppressed). We excluded subsequent encounters during the study period. Descriptive statistics summarized the prevalence of diagnostic criteria and risk factors for CDI. Comparisons between groups were performed using Mann Whitney U, Chi-square, or Fisher’s exact tests.
Results: Among 187 patients, we did not observe a significant difference between test groups in the percentage of patients who had ≥1 diagnostic criterion (85.7% vs 78.3%; p=.6) or risk factor (66.7% vs 83.7%; p=.06) for CDI (Fig. 2). We did not observe a significant association between having ≥1 diagnostic criterion (88.1% vs 89.7%; p=.9) or risk factor (83.6% vs 87.2%; p=.6) for CDI and coinfection with another pathogen (Fig. 2). We also did not observe a significant difference in time to diagnosis (2.1 vs. 2.0 hours; p=.8) or treatment (7.5 vs 5.8 hours; p=.06) between those who had NAAT + GIP testing and those who had NAAT testing only (Fig. 2).Conclusion(s): Suppression of the GIP C. difficile result may be beneficial for diagnostic stewardship as the presence of CDI risk factors or diagnostic criteria did not differ between patients with a visible or suppressed C. difficile result. Importantly, in our study result suppression did not necessarily result in delays in diagnosis or treatment of CDI.
Figure 1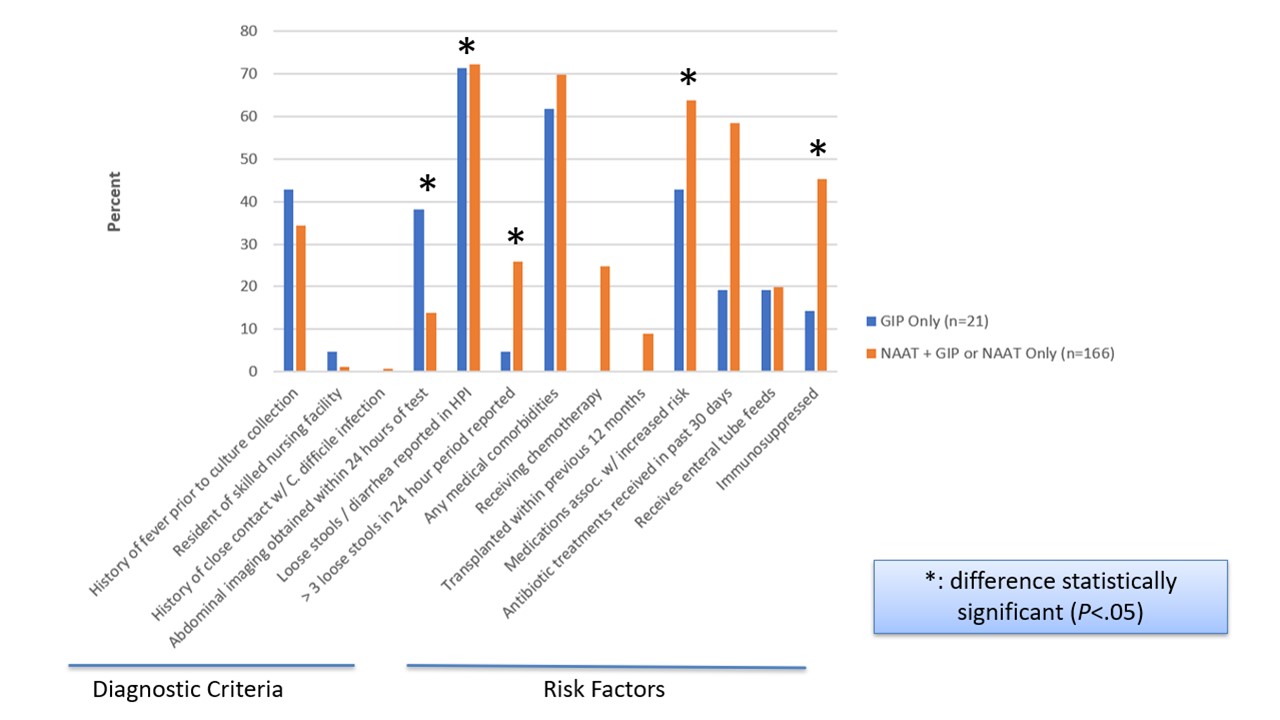 Diagnostic criteria and risk factors for C. difficile infection by targeted C. difficile test status
Diagnostic criteria and risk factors for C. difficile infection by targeted C. difficile test status
Figure 2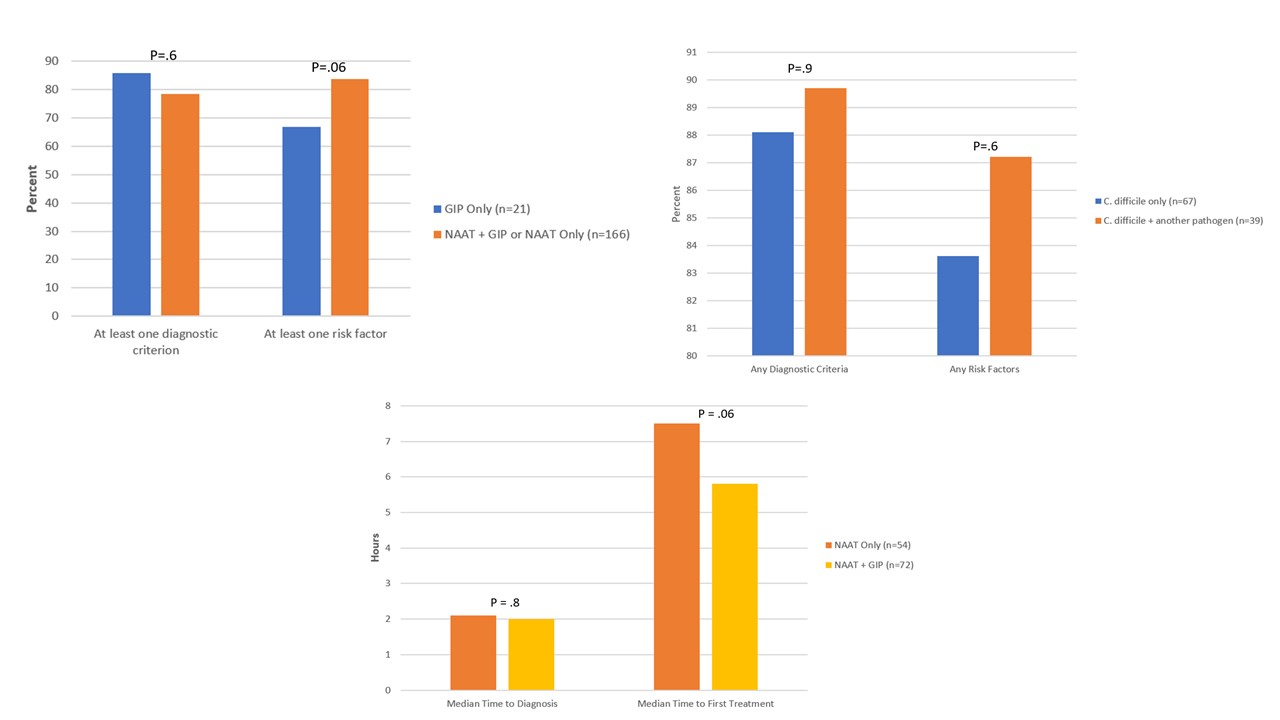 Diagnostic criteria and risk factors for C. difficile infection by targeted C. difficile test status; Diagnostic criteria and risk factors for C. difficile infection by coinfection status among patients with NAAT+GIP or GIP testing only; Time to diagnosis and treatment among patients treated for C. difficile infection by type of C. difficile test
Diagnostic criteria and risk factors for C. difficile infection by targeted C. difficile test status; Diagnostic criteria and risk factors for C. difficile infection by coinfection status among patients with NAAT+GIP or GIP testing only; Time to diagnosis and treatment among patients treated for C. difficile infection by type of C. difficile test
Objective: Among patients with positive C. difficile testing (NAAT +/- GIP), we aimed to determine if the presence of CDI diagnostic criteria and risk factors are associated with 1) obtaining targeted C. difficile NAAT testing or 2) coinfection with C. difficile and other pathogen and 3) if time to diagnosis and time to treatment differed between patients with CDI receiving both NAAT and GIP compared to those receiving NAAT testing alone.
Design/Methods: We performed a retrospective single center study from 2/2/2016 to 12/17/2018 of inpatient and outpatients with positive C. difficile test result (NAAT or GIP, Fig. 1). The primary study exposure was obtaining targeted C. difficile testing (NAAT +/- GIP; visible) or not (GIP alone; suppressed). We excluded subsequent encounters during the study period. Descriptive statistics summarized the prevalence of diagnostic criteria and risk factors for CDI. Comparisons between groups were performed using Mann Whitney U, Chi-square, or Fisher’s exact tests.
Results: Among 187 patients, we did not observe a significant difference between test groups in the percentage of patients who had ≥1 diagnostic criterion (85.7% vs 78.3%; p=.6) or risk factor (66.7% vs 83.7%; p=.06) for CDI (Fig. 2). We did not observe a significant association between having ≥1 diagnostic criterion (88.1% vs 89.7%; p=.9) or risk factor (83.6% vs 87.2%; p=.6) for CDI and coinfection with another pathogen (Fig. 2). We also did not observe a significant difference in time to diagnosis (2.1 vs. 2.0 hours; p=.8) or treatment (7.5 vs 5.8 hours; p=.06) between those who had NAAT + GIP testing and those who had NAAT testing only (Fig. 2).Conclusion(s): Suppression of the GIP C. difficile result may be beneficial for diagnostic stewardship as the presence of CDI risk factors or diagnostic criteria did not differ between patients with a visible or suppressed C. difficile result. Importantly, in our study result suppression did not necessarily result in delays in diagnosis or treatment of CDI.
Figure 1
 Diagnostic criteria and risk factors for C. difficile infection by targeted C. difficile test status
Diagnostic criteria and risk factors for C. difficile infection by targeted C. difficile test statusFigure 2
 Diagnostic criteria and risk factors for C. difficile infection by targeted C. difficile test status; Diagnostic criteria and risk factors for C. difficile infection by coinfection status among patients with NAAT+GIP or GIP testing only; Time to diagnosis and treatment among patients treated for C. difficile infection by type of C. difficile test
Diagnostic criteria and risk factors for C. difficile infection by targeted C. difficile test status; Diagnostic criteria and risk factors for C. difficile infection by coinfection status among patients with NAAT+GIP or GIP testing only; Time to diagnosis and treatment among patients treated for C. difficile infection by type of C. difficile test