Pediatric Nutrition
Category: Abstract Submission
Pediatric Nutrition I
247 - Effects of a new infant formula with a novel high-quality protein matrix and whole milk on energetic efficiency and gastrointestinal tolerance
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 247
Publication Number: 247.141
Publication Number: 247.141
Devon Kuehn, ByHeart, Inc, New York, NY, United States; Steven H. Zeisel, UNC Chapel Hill, Chapel Hill, NC, United States; Diana F. Orenstein, ByHeart, Inc., New York, NY, United States; J. Bruce German, University of california, Davis, Davis, CA, United States; Catherine J. Field, University of Alberta Faculty of Medicine and Dentistry, Edmonton, AB, Canada; Sharon M. Donovan, University of Illinois, Urbana-Champaign, Urbana, IL, United States; Bo Lönnerdal, University of California Davis, Davis, CA, United States

Devon Kuehn, MD (she/her/hers)
Chief Medical Officer
ByHeart, Inc
New York, New York, United States
Presenting Author(s)
Background: Infant formula (IF) often contains protein at levels above human milk (HM) to ensure adequate amino acid provision and to compensate for lower bioavailability. Consequentially, protein intake of formula-fed infants exceeds that of HM-fed infants. Protein overfeeding is implicated in negative outcomes and recent studies have concluded the key to reducing protein intake, and therefore improving outcomes, is protein quality.
Objective: A new IF containing a novel protein matrix (with alpha-lactalbumin, lactoferrin, and partially hydrolyzed whey proteins) and whole milk was compared to a commercially available IF and HM to evaluate growth, safety, and tolerance.
Design/Methods: This randomized, double-blind, multi-center trial included healthy, term infants, birth weight of ≥ 2500g, ≤ 14d, fifth to ninety-fifth percentile of WHO Growth Standards. Infants were randomized to receive either the study formula (SF) or an isoenergetic IF with identical total protein content (CF). Primary outcome was mean daily weight gain. Secondary outcomes were anthropometrics, amino acid levels, formula intake, adverse events, gastrointestinal characteristics, and general disposition. An a priori determined sample size of 70 infants per formula group would be sufficient to demonstrate non-inferiority in weight gain velocity, based on the established margin of -3 g/d.
Results: Statistical analysis included the Per-Protocol Population (61 SF, 67 CF) and a predominantly HM-fed group (57). Demographics were similar. There were no differences between the formula groups for z-scores over time. Formula intake (-0.33 oz/kg/d, 95 CI: -0.66, -0.01, p=0.05) and mean protein intake (-0.13 g/kg/d, 95 CI: -0.26, 0.00, p=0.05) were lower in the SF infants, with higher serum essential amino acid concentrations (including tryptophan) (Table 1) compared to the CF infants. Energetic efficiency was 14.0% (95 CI: 8.3%, 19.7%), 13.0% (95 CI: 6.0%, 20.0%), and 18.1% (95 CI: 9.4%, 26.8%) higher for weight, length, and head circumference, respectively, in SF infants compared to the CF infants. (Figure 1) SF infants had fewer spit-ups (p=0.01) and softer mean stool consistency (0.39, 95 CI 0.27, 0.52, p< 0.001) than CF infants. (Figure 2)Conclusion(s): The SF, containing a high-quality protein matrix and whole milk, decreased parent-reported gastrointestinal measurements, and supported more efficient growth and improved protein utilization with less daily formula and protein intake. Reducing the metabolic burden of protein overfeeding associated with infant formula has the potential to improve outcomes to benefit infants who do not have access to HM.
Table 1: Serum Essential and Conditionally Essential Amino Acid Concentrations and Ratios (µmol/l) at Week 16 by Feeding Group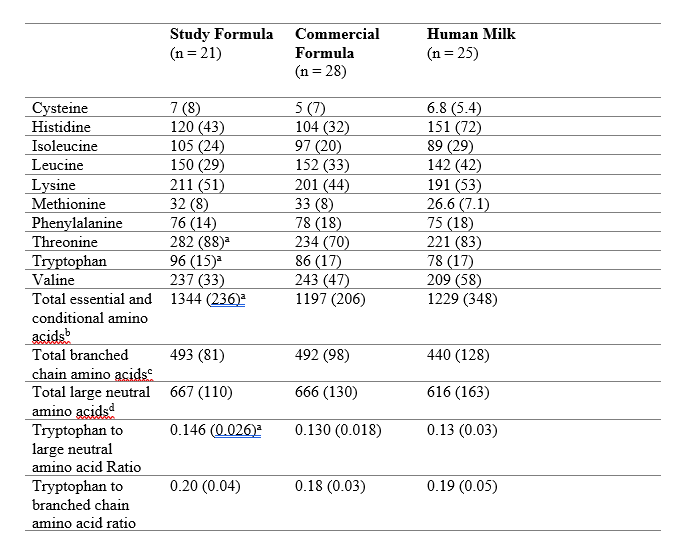 Mean (SD)
Mean (SD)
aSignificant difference between SF and CF, p < 0.05
bThe essential and conditionally essential amino acid group was the sum of cysteine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine
cThe branched-chain group was the sum of isoleucine, leucine, and valine.
dThe large neutral amino acid (LNAA) group was the sum of isoleucine, leucine, phenylalanine, tyrosine, valine.
Figure 1: Model-based Mean Energetic Efficiency by Feeding Group and Study Visit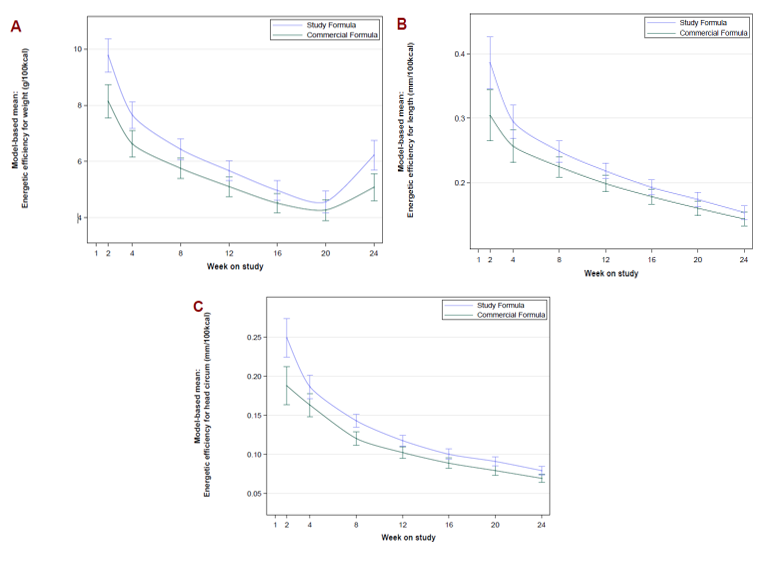 Results from MMRM using sex, age at enrollment, formula, site, visit, formula by site interaction, formula by visit interaction, weight, length or head circumference at baseline, and food intake to account for introduction of supplementary solid food introduced during the study period as factors; weight (A), length (B), HC (C).
Results from MMRM using sex, age at enrollment, formula, site, visit, formula by site interaction, formula by visit interaction, weight, length or head circumference at baseline, and food intake to account for introduction of supplementary solid food introduced during the study period as factors; weight (A), length (B), HC (C).
Objective: A new IF containing a novel protein matrix (with alpha-lactalbumin, lactoferrin, and partially hydrolyzed whey proteins) and whole milk was compared to a commercially available IF and HM to evaluate growth, safety, and tolerance.
Design/Methods: This randomized, double-blind, multi-center trial included healthy, term infants, birth weight of ≥ 2500g, ≤ 14d, fifth to ninety-fifth percentile of WHO Growth Standards. Infants were randomized to receive either the study formula (SF) or an isoenergetic IF with identical total protein content (CF). Primary outcome was mean daily weight gain. Secondary outcomes were anthropometrics, amino acid levels, formula intake, adverse events, gastrointestinal characteristics, and general disposition. An a priori determined sample size of 70 infants per formula group would be sufficient to demonstrate non-inferiority in weight gain velocity, based on the established margin of -3 g/d.
Results: Statistical analysis included the Per-Protocol Population (61 SF, 67 CF) and a predominantly HM-fed group (57). Demographics were similar. There were no differences between the formula groups for z-scores over time. Formula intake (-0.33 oz/kg/d, 95 CI: -0.66, -0.01, p=0.05) and mean protein intake (-0.13 g/kg/d, 95 CI: -0.26, 0.00, p=0.05) were lower in the SF infants, with higher serum essential amino acid concentrations (including tryptophan) (Table 1) compared to the CF infants. Energetic efficiency was 14.0% (95 CI: 8.3%, 19.7%), 13.0% (95 CI: 6.0%, 20.0%), and 18.1% (95 CI: 9.4%, 26.8%) higher for weight, length, and head circumference, respectively, in SF infants compared to the CF infants. (Figure 1) SF infants had fewer spit-ups (p=0.01) and softer mean stool consistency (0.39, 95 CI 0.27, 0.52, p< 0.001) than CF infants. (Figure 2)Conclusion(s): The SF, containing a high-quality protein matrix and whole milk, decreased parent-reported gastrointestinal measurements, and supported more efficient growth and improved protein utilization with less daily formula and protein intake. Reducing the metabolic burden of protein overfeeding associated with infant formula has the potential to improve outcomes to benefit infants who do not have access to HM.
Table 1: Serum Essential and Conditionally Essential Amino Acid Concentrations and Ratios (µmol/l) at Week 16 by Feeding Group
 Mean (SD)
Mean (SD)aSignificant difference between SF and CF, p < 0.05
bThe essential and conditionally essential amino acid group was the sum of cysteine, histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine
cThe branched-chain group was the sum of isoleucine, leucine, and valine.
dThe large neutral amino acid (LNAA) group was the sum of isoleucine, leucine, phenylalanine, tyrosine, valine.
Figure 1: Model-based Mean Energetic Efficiency by Feeding Group and Study Visit
 Results from MMRM using sex, age at enrollment, formula, site, visit, formula by site interaction, formula by visit interaction, weight, length or head circumference at baseline, and food intake to account for introduction of supplementary solid food introduced during the study period as factors; weight (A), length (B), HC (C).
Results from MMRM using sex, age at enrollment, formula, site, visit, formula by site interaction, formula by visit interaction, weight, length or head circumference at baseline, and food intake to account for introduction of supplementary solid food introduced during the study period as factors; weight (A), length (B), HC (C).