Neonatal Hematology & Bilirubin Metabolism
Category: Abstract Submission
Neonatal Hematology & Bilirubin Metabolism II
351 - Evaluation of Intravenous Immunoglobulin Administration for Hyperbilirubinemia in Newborn Infants with Hemolytic Disease
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 351
Publication Number: 351.123
Publication Number: 351.123
Daniel Mohan, UH Rainbow Babies & Children's Hospital, Cleveland, OH, United States; Hannah Lu, UH Rainbow Babies & Children's Hospital, Cleveland, OH, United States; Jaime Marasch, UH Rainbow Babies & Children's Hospital, Cleveland, OH, United States; Jacquelyn McClary, UH Rainbow Babies & Children's Hospital, Richfield, OH, United States; Mary Nock, UH Rainbow Babies & Children's Hospital, Cleveland, OH, United States; Rita M. Ryan, Case Western Reserve University, Cleveland, OH, United States

Daniel Mohan, MD
PGY-2 Resident
UH Rainbow Babies & Children's Hospital
Cleveland, Ohio, United States
Presenting Author(s)
Background:
American Academy of Pediatrics guidelines (2004) recommend the use of intravenous gamma globulin (IVIG) for infants with immune-mediated hemolysis and severe hyperbilirubinemia who are within 2-3 of double-volume exchange transfusion (DVET) level despite use of intensive phototherapy. The randomized trials demonstrating efficacy for IVIG were in Coombs positive infants with Rh or ABO hemolytic disease of the newborn. With the AAP recommendation, clinicians have been using IVIG outside of the specific parameters despite the known adverse event of IVIG-induced hemolysis, the hospital cost for a 3 kg infant being $684 and patient charge $3,375, and the potential for significant donor exposure (1,000- 15,000 per does depending on the preparation) In addition, recently the efficacy of IVIG to prevent DVET has been debated.
Objective: To determine the use of IVIG in a single center NICU over a give-year period with regard to meeting the AAP guidelines.
Design/Methods: Pharmacy records were used to identify all NICU patients who had IVIG ordered. There were 73 patients with orders; 71 actually received IVIG. Twelve babies received IVIG for indications other than immune mediated hemolysis/ hyperbilirubinemia (Figure). The remaining 59 babies had detailed data collection.
Results:
The median gestational age was 39 weeks, 53% were male; 78% were black; 47 (78%) were an ABO “set-up” and 11 (19%) had an Rh setup; 42 (71%) had a positive Coombs test (Table 1). The median age at which phototherapy was started was 7 hours, with median duration 86 hours. Fourteen (24%) of the 59 babies received a 2nd dose of IVIG and 2 had a 3rd dose ordered with one receiving (Table 2). The median dose was 1 g/kg. The median hours of age IVIG was received were 12.6, 40.4 and 79.5 hours for the 1st, 2nd and 3rd doses. The only adverse event noted was hypoglycemia in 2 babies during the infusion. Of the 59 babies, only 12% had a total serum bilirubin with 3 of exchange level; the median hours between phototherapy started and IVIG ordered was 2.6 hours, mean 7 hours; 66% were on phototherapy for < 4 hours prior to IVIG being ordered. We considered meeting the AAP guidelines as having a bilirubin with 3 of exchange level, having a positive Coombs test and having had at least four hours of intensive phototherapy. Meeting all criteria for IVIG was rare (Figure). In particular only 11.9% of the time was the bilirubin level with 3 of the exchange level.
Conclusion(s):
These data suggest our clinicians are often using IVIG outside of the AAP recommendations. We are developing a clinical practice guideline for our unit.
Table 1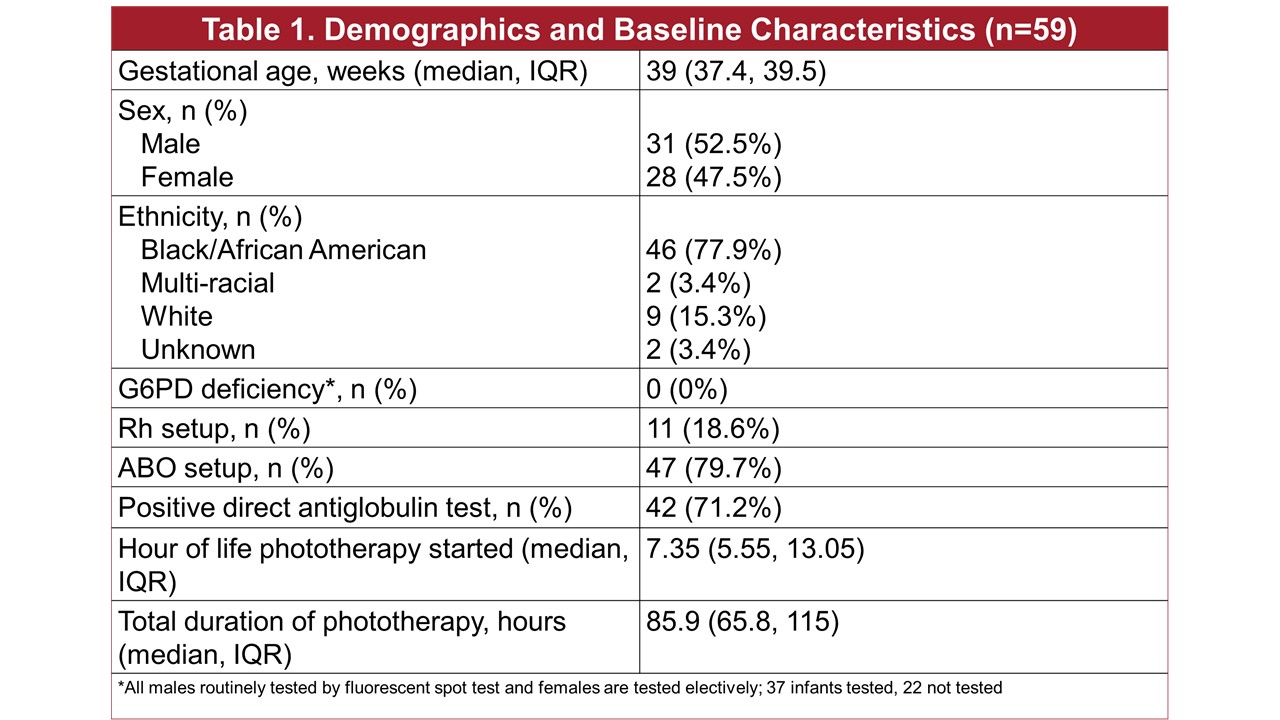
Table 2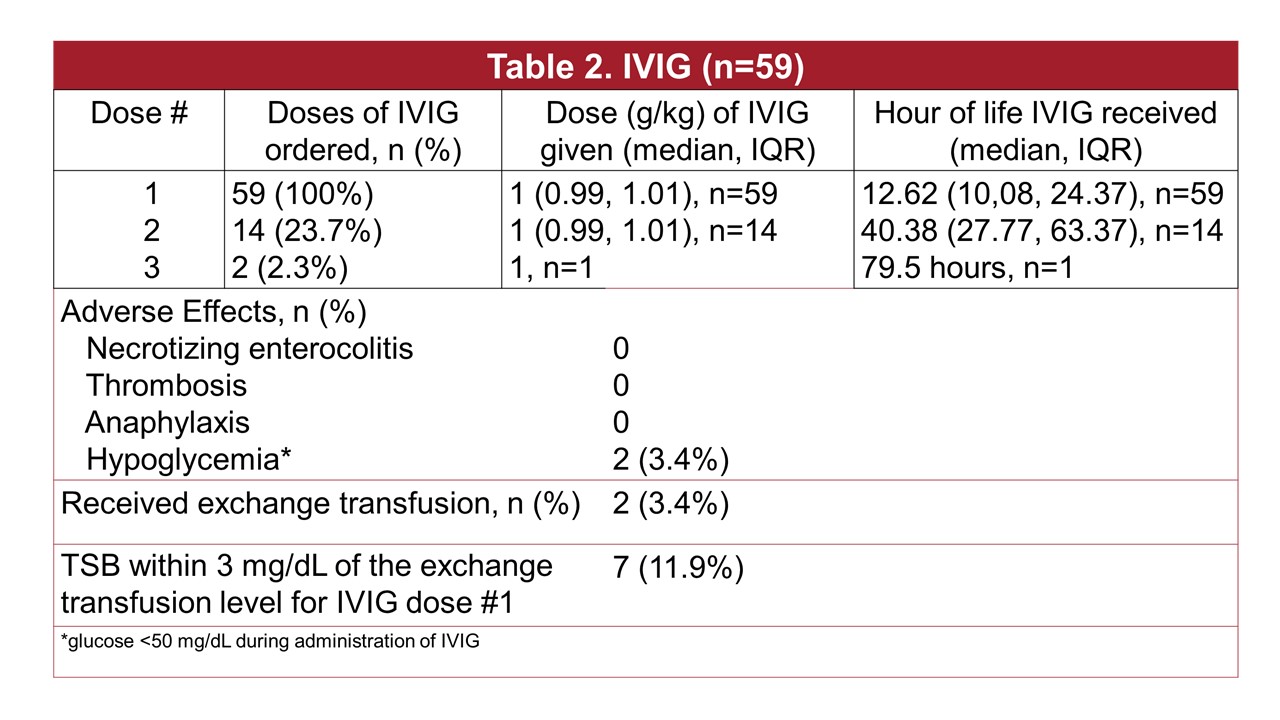
American Academy of Pediatrics guidelines (2004) recommend the use of intravenous gamma globulin (IVIG) for infants with immune-mediated hemolysis and severe hyperbilirubinemia who are within 2-3 of double-volume exchange transfusion (DVET) level despite use of intensive phototherapy. The randomized trials demonstrating efficacy for IVIG were in Coombs positive infants with Rh or ABO hemolytic disease of the newborn. With the AAP recommendation, clinicians have been using IVIG outside of the specific parameters despite the known adverse event of IVIG-induced hemolysis, the hospital cost for a 3 kg infant being $684 and patient charge $3,375, and the potential for significant donor exposure (1,000- 15,000 per does depending on the preparation) In addition, recently the efficacy of IVIG to prevent DVET has been debated.
Objective: To determine the use of IVIG in a single center NICU over a give-year period with regard to meeting the AAP guidelines.
Design/Methods: Pharmacy records were used to identify all NICU patients who had IVIG ordered. There were 73 patients with orders; 71 actually received IVIG. Twelve babies received IVIG for indications other than immune mediated hemolysis/ hyperbilirubinemia (Figure). The remaining 59 babies had detailed data collection.
Results:
The median gestational age was 39 weeks, 53% were male; 78% were black; 47 (78%) were an ABO “set-up” and 11 (19%) had an Rh setup; 42 (71%) had a positive Coombs test (Table 1). The median age at which phototherapy was started was 7 hours, with median duration 86 hours. Fourteen (24%) of the 59 babies received a 2nd dose of IVIG and 2 had a 3rd dose ordered with one receiving (Table 2). The median dose was 1 g/kg. The median hours of age IVIG was received were 12.6, 40.4 and 79.5 hours for the 1st, 2nd and 3rd doses. The only adverse event noted was hypoglycemia in 2 babies during the infusion. Of the 59 babies, only 12% had a total serum bilirubin with 3 of exchange level; the median hours between phototherapy started and IVIG ordered was 2.6 hours, mean 7 hours; 66% were on phototherapy for < 4 hours prior to IVIG being ordered. We considered meeting the AAP guidelines as having a bilirubin with 3 of exchange level, having a positive Coombs test and having had at least four hours of intensive phototherapy. Meeting all criteria for IVIG was rare (Figure). In particular only 11.9% of the time was the bilirubin level with 3 of the exchange level.
Conclusion(s):
These data suggest our clinicians are often using IVIG outside of the AAP recommendations. We are developing a clinical practice guideline for our unit.
Table 1

Table 2

