Pediatric Nutrition
Category: Abstract Submission
Pediatric Nutrition I
250 - Growth and Gastrointestinal Tolerance in Healthy Term Infants Fed Milk-Based Infant Formula supplemented with Five Human Milk Oligosaccharides (HMOs).
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 250
Publication Number: 250.141
Publication Number: 250.141
John Lasekan, Abbott Nutrition, Columbus, OR, United States; Yong Choe, Abbott Laboratories, Columbus, OH, United States; Svyatoslav V. Dvoretskiy, Abbott Nutrition, Columbus, OH, United States; Amy A. Devitt-Maicher, Abbott Nutrition, Columbus, OH, United States; Sue Zhang, Abbott Nutrition, COlumbus, OH, United States; Amy Mackey, Abbott Nutrition, Columbus, OH, United States; Christine Steele, abbott, Columbus, OH, United States; Michelle Johnson, Abbott Labs, Columbus, OH, United States; Maria E. Geraldine, Abbott Laboratories, Columbus, OH, United States

John Lasekan, PhD
Principal Research Scientist
Abbott Nutrition
Columbus, Oregon, United States
Presenting Author(s)
Background: Human milk (HM) is the gold standard nutrition source for infants’ growth and development and provides important prebiotic human milk oligosaccharides (HMOs). The 5 most abundant HMOs (out of > 150 identified) are 2’-fucosyllactose (2’-FL), lacto-N-tetraose (LNT), 3-fucosyllactose (3-FL), 6’-sialyllactose (6’-SL), and 3’-sialyllactose (3’-SL). Infant formulas supplemented with these 5 HMOs need clinical assessment.
Objective: The goal of this study was to access the supplemental impact of 5 HMOs on growth, gastrointestinal (GI) tolerance and health outcomes in formula-fed infants.
Design/Methods: A randomized, double-blind, controlled parallel feeding growth trial was conducted in 0 to 14 days old healthy term infants weighing ≥2490g. Infants were randomized to either a control milk-based formula (CF; n=129), or experimental milk-based formula (EF; n=130) containing 5 HMOs (5.75 g/L; 2′-FL, 3-FL, LNT, 3′-SL and 6′-SL) and fed until 119 days of age. A HM fed reference group (HM; n=104) was assessed concurrently. The primary study outcome was weight gain from 14 to 119 days of age. Growth trajectories during COVID-19 versus combined Non-COVID-19 and COVID-19 periods were described using the Gompertz model. The study was approved by the ethics committee.
Results: No significant differences were observed among the 3 study groups for weight gain per day from 14 to 119 days of age, irrespective of COVID-19 or combined Non-COVID-19 and COVID-19 periods’ assessments (p=0.337; Protocol evaluable, PE). The analysis also confirmed the non-inferiority of EF relative to CF, using a margin of 3 g/day weight gain difference. No significant differences (p>0.05) were noted among the 3 groups for gains in weight, length, and head circumference (HC) from 14 to 119 days of age. The EF group had more stools that were soft, frequent and yellowish, and were lower in constipation dimension than the CF group, and were rated more similarly to those from the HM group compared to the CF group. CF-fed infants compared to EF-fed infants were more seen by health care professionals for illness from study entry to 56 (p=0.044; CF=11%, EF=2.9%) and 84 days of age (p=0.028; CF=12%, EF=2.9%). There was no significant difference (p>0.05) in the occurrence of adverse events (AEs) and serious adverse events (SAEs) among the 3 groups (AEs; CF=31.7%, EF=32.0%, HM=26.5% and SAEs; CF=2.4%, EF=0.8%, HM=2.9%).Conclusion(s): This study demonstrated that the experimental formula containing a blend of 5 HMOs supported normal growth, GI tolerance and a safe use in healthy term infants. Study registered at clinicaltrials.gov (NCT04105686).
WHO Growth Charts for Male and Female Subjects in the Protocol Evaluable (PE) Cohorts.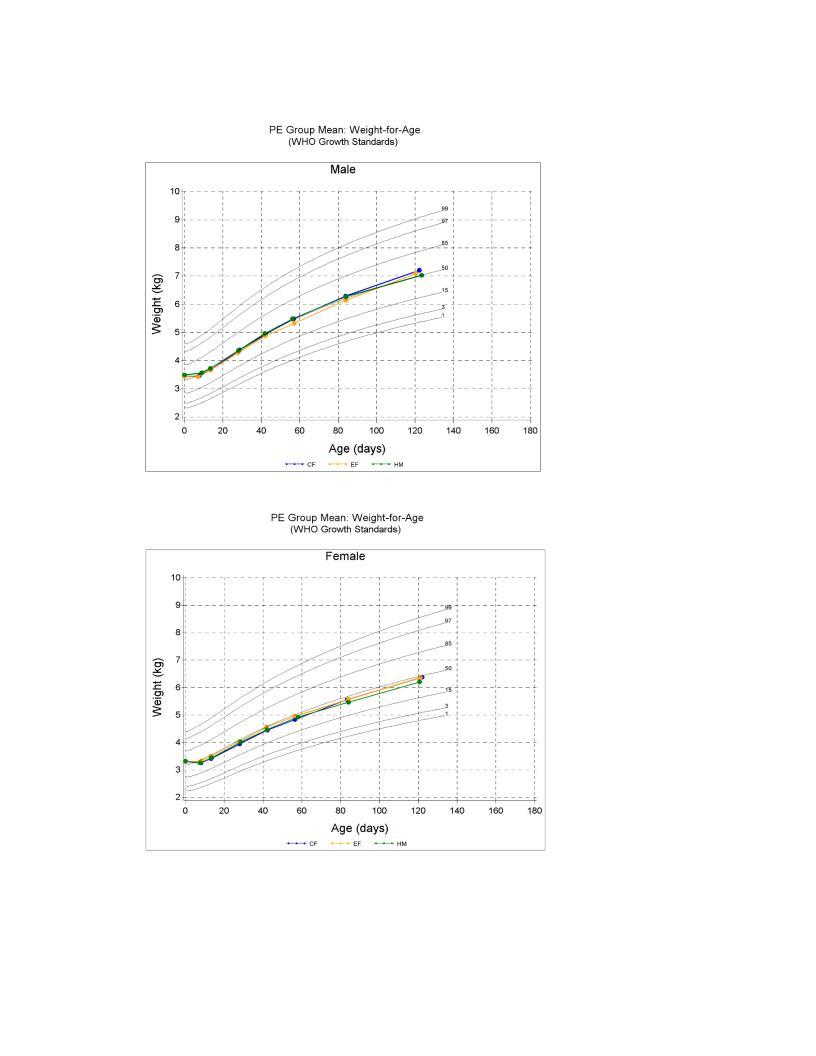
Objective: The goal of this study was to access the supplemental impact of 5 HMOs on growth, gastrointestinal (GI) tolerance and health outcomes in formula-fed infants.
Design/Methods: A randomized, double-blind, controlled parallel feeding growth trial was conducted in 0 to 14 days old healthy term infants weighing ≥2490g. Infants were randomized to either a control milk-based formula (CF; n=129), or experimental milk-based formula (EF; n=130) containing 5 HMOs (5.75 g/L; 2′-FL, 3-FL, LNT, 3′-SL and 6′-SL) and fed until 119 days of age. A HM fed reference group (HM; n=104) was assessed concurrently. The primary study outcome was weight gain from 14 to 119 days of age. Growth trajectories during COVID-19 versus combined Non-COVID-19 and COVID-19 periods were described using the Gompertz model. The study was approved by the ethics committee.
Results: No significant differences were observed among the 3 study groups for weight gain per day from 14 to 119 days of age, irrespective of COVID-19 or combined Non-COVID-19 and COVID-19 periods’ assessments (p=0.337; Protocol evaluable, PE). The analysis also confirmed the non-inferiority of EF relative to CF, using a margin of 3 g/day weight gain difference. No significant differences (p>0.05) were noted among the 3 groups for gains in weight, length, and head circumference (HC) from 14 to 119 days of age. The EF group had more stools that were soft, frequent and yellowish, and were lower in constipation dimension than the CF group, and were rated more similarly to those from the HM group compared to the CF group. CF-fed infants compared to EF-fed infants were more seen by health care professionals for illness from study entry to 56 (p=0.044; CF=11%, EF=2.9%) and 84 days of age (p=0.028; CF=12%, EF=2.9%). There was no significant difference (p>0.05) in the occurrence of adverse events (AEs) and serious adverse events (SAEs) among the 3 groups (AEs; CF=31.7%, EF=32.0%, HM=26.5% and SAEs; CF=2.4%, EF=0.8%, HM=2.9%).Conclusion(s): This study demonstrated that the experimental formula containing a blend of 5 HMOs supported normal growth, GI tolerance and a safe use in healthy term infants. Study registered at clinicaltrials.gov (NCT04105686).
WHO Growth Charts for Male and Female Subjects in the Protocol Evaluable (PE) Cohorts.

