Adolescent Medicine: General
Category: Abstract Submission
Adolescent Medicine II
499 - HPV Vaccination Before and During the COVID-19 Pandemic
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 499
Publication Number: 499.100
Publication Number: 499.100
Jenny Francis, University of Texas Southwestern Medical School, Dallas, TX, United States; Sitara Weerakoon, University of Texas Health Science Center, Dallas, TX, United States; Stephanie E. Trenkner, Children's Health, Indianapolis, IN, United States; Julia C. Durante, University of Texas Southwestern Medical School, Dallas, TX, United States; Sonia Allouch, Children’s Medical Center Dallas, University of Texas Southwestern, Dallas, TX, United States; Matthew S. Mathew, University of Texas Health Science Center at Houston, Dallas, TX, United States; Serena L. Lucas, Children's Health System of Texas, Gainesville, GA, United States; Jasmin A. Tiro, UT Southwestern Medical Center, Dallas, TX, United States

Jenny Francis, MD, MPH (she/her/hers)
Associate Professor
University of Texas Southwestern Medical School
Dallas, Texas, United States
Presenting Author(s)
Background: The COVID-19 pandemic disrupted the delivery of vaccine services.
Objective: We aimed to characterize human papillomavirus (HPV) vaccine administration by age from 2019 (pre-pandemic), through 2020 and 2021 (pandemic years) and compare HPV vaccination by seasons to identify data informed vaccine recall strategies.
Design/Methods: This observational, cross-sectional study assessed a large cohort of adolescent patients at a tertiary care, academic hospital primary care setting from January 2019 through September 2021. “Opportunities achieved” to receive the HPV vaccine were crossed with demographic characteristics (age, gender, ethnicity/race, and insurance satus) using bivariate tests. Difference-in-difference tests compared total vaccines administered per period (season) between each year to account for seasonal variation, secular trends and mirror the pandemic “seasons” of winter: pre-pandemic (January 1 – March 11), spring: stay-at-home (March 12 – May 31) and summer: re-opening (June 1- August 31). Each season was compared to the same period in 2019, 2020 and 2021.
Results: Of the 9,362 eligible encounters, the 9-10 year-old age group had the lowest vaccination compared to other age groups (p < 0.001). There were no detectable difference by gender or insurance status. Overall, there were more who got the HPV vaccine in 2021 (37.3%) than 2020 (35.6%) or 2019 (30.9%) (p < 0.001). When aggregating seasons from 2019 – 2021 across all age groups, the summer (June – August) had the highest percentage (37.9%) who received the vaccine compared to spring (35.6%) or winter (30.9%) (p = 0.01). When comparing 2020 to the same periods in 2019, adjusted percent change (RRR; ratio of rate ratios) across seasons showed an initial increase in the winter followed by a decrease in the spring and summer (+18% winter, -37% spring, -22% summer). Comparing 2021 to 2020, there were smaller decreases with a large incraese in the spring (-12% winter, +45% spring, -4% summer). Comparing 2021 to 2019, there was a small increase in the winter followed by decreases in the spring and summer (+4% in winter, -9% spring, -25% summer).Conclusion(s): In this sample, the HPV vaccine numbers of 2021 are not back to pre-pandemic 2019 levels. Recall efforts should focus on the 9-10 year-olds during spring and summer seasons where 2021 vaccination has not caught-up to previous levels.
Acknowledgements: American Cancer Society HPV Cancer Free Texas, National Institutes of Health (K23 HD097291)
Table 1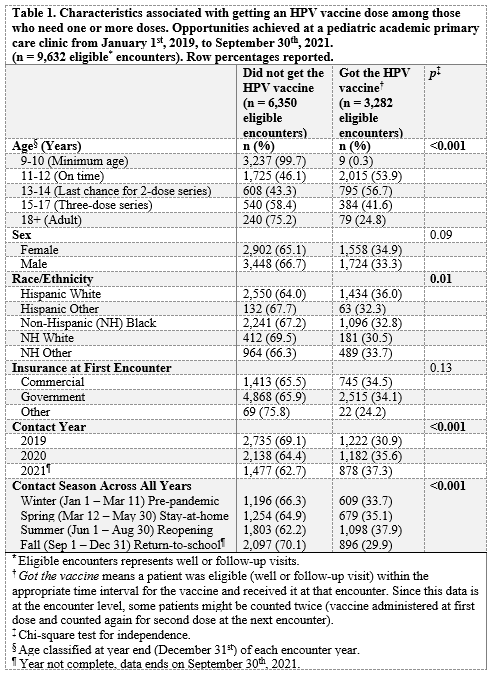
Table 2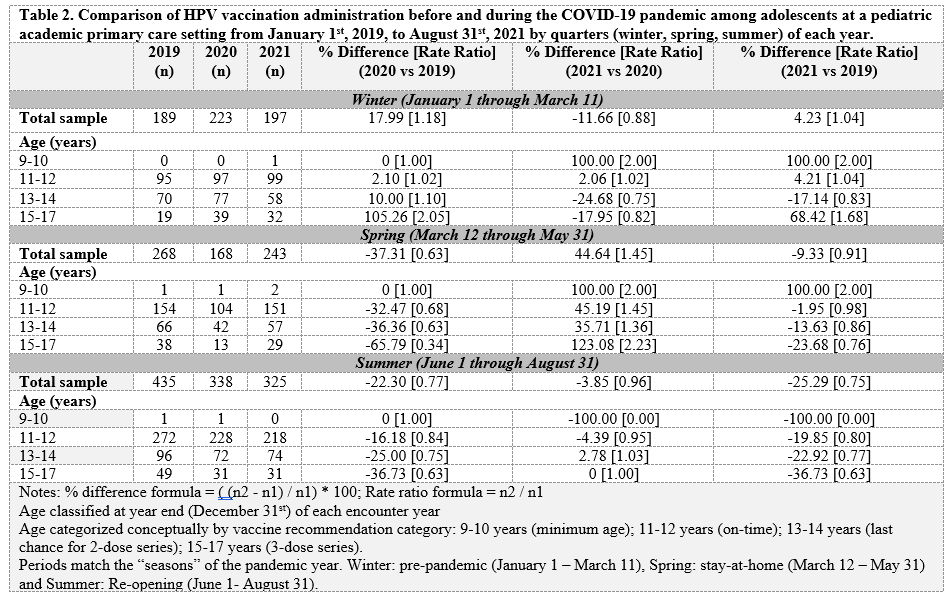
Objective: We aimed to characterize human papillomavirus (HPV) vaccine administration by age from 2019 (pre-pandemic), through 2020 and 2021 (pandemic years) and compare HPV vaccination by seasons to identify data informed vaccine recall strategies.
Design/Methods: This observational, cross-sectional study assessed a large cohort of adolescent patients at a tertiary care, academic hospital primary care setting from January 2019 through September 2021. “Opportunities achieved” to receive the HPV vaccine were crossed with demographic characteristics (age, gender, ethnicity/race, and insurance satus) using bivariate tests. Difference-in-difference tests compared total vaccines administered per period (season) between each year to account for seasonal variation, secular trends and mirror the pandemic “seasons” of winter: pre-pandemic (January 1 – March 11), spring: stay-at-home (March 12 – May 31) and summer: re-opening (June 1- August 31). Each season was compared to the same period in 2019, 2020 and 2021.
Results: Of the 9,362 eligible encounters, the 9-10 year-old age group had the lowest vaccination compared to other age groups (p < 0.001). There were no detectable difference by gender or insurance status. Overall, there were more who got the HPV vaccine in 2021 (37.3%) than 2020 (35.6%) or 2019 (30.9%) (p < 0.001). When aggregating seasons from 2019 – 2021 across all age groups, the summer (June – August) had the highest percentage (37.9%) who received the vaccine compared to spring (35.6%) or winter (30.9%) (p = 0.01). When comparing 2020 to the same periods in 2019, adjusted percent change (RRR; ratio of rate ratios) across seasons showed an initial increase in the winter followed by a decrease in the spring and summer (+18% winter, -37% spring, -22% summer). Comparing 2021 to 2020, there were smaller decreases with a large incraese in the spring (-12% winter, +45% spring, -4% summer). Comparing 2021 to 2019, there was a small increase in the winter followed by decreases in the spring and summer (+4% in winter, -9% spring, -25% summer).Conclusion(s): In this sample, the HPV vaccine numbers of 2021 are not back to pre-pandemic 2019 levels. Recall efforts should focus on the 9-10 year-olds during spring and summer seasons where 2021 vaccination has not caught-up to previous levels.
Acknowledgements: American Cancer Society HPV Cancer Free Texas, National Institutes of Health (K23 HD097291)
Table 1

Table 2

