Neonatal General
Category: Abstract Submission
Neonatology General 3
375 - Impact of a Revised State of Illinois Protocol for Newborn Screening in the NICU
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 375
Publication Number: 375.133
Publication Number: 375.133
Mohammad Jaber, SIU School of Medicine, Springfield, IL, United States; Kristen Cecil, Southern Illinois University School of Medicine, Springfield, IL, United States; Dharmendra J. Nimavat, Southern Illinois University School of Medicine, SPRINGFIELD, IL, United States; Beau Batton, SIU SOM, SPRINGFIELD, IL, United States; Albert Botchway, Southern Illinois University School of Medicine, Springfield, IL, United States; Mohamed Ahamed, Southern Illinois University School of Medicine, Springfield, IL, United States
- MJ
Mohammad Jaber, MD
PGY3 Pediatric Resident
SIU School of Medicine
Springfield, Illinois, United States
Presenting Author(s)
Background: Hospitals in Illinois are mandated to perform newborn blood screening (NBS) for healthy newborns at 24 hours of age to aid with early identification of 64 congenital disorders. In May, 2019 Illinois recommended the following practice change for NICU patients: perform NBS upon NICU admission (irrespective of postnatal age), at 72 hours of age, and again at either 28 days or hospital discharge (whichever is earlier). Our previous practice was to obtain a NBS at 24 hours and 14 days.
Objective: To assess the impact of change in the timing of NBS on the number of NBS performed, number of true and false positive test results, and associated cost of NBS.
Design/Methods: Retrospective cohort study performed at a level III NICU serving as a regional perinatal center for central and southern Illinois (ADC: 40, >700 annual admissions). Data was collected for inborn NICU admissions from two cohorts: 04/2018 – 04/2019 (historical cohort) and 05/2019 – 05/2020 (study cohort). Demographic data and NBS testing data were collected. Chi square was used for statistical analysis with p < 0.05 considered significant.
Results: 2946 NBS were performed on 1305 patients (median: two NBS/patient, range: one - four), including 1313 NBS performed on 670 patients in the historical cohort and 1633 NBS performed on 635 patients in the study cohort (table). Demographics data were similar between cohorts. Abnormal NBS were significantly more common after implementation of the practice change (20% vs 30%, OR: 1.72, 95% CI: 1.44-2.05, p < 0.001). Abnormal NBS results for congenital hypothyroidism, cystic fibrosis, and amino acidopathies were more frequent in the study cohort, but the incidence of abnormal NBS results for the other congenital conditions were similar. More follow-up testing/evaluation was required for the study vs historical cohort at an additional cost of ~$32,001 ($9,391 vs $26,392 for additional testing plus $15,000 for an addition 0.3 FTE for test tracking), but this was not associated with a statistically significant change in the number true positive NBS results ( < 1% for both cohorts).Conclusion(s): In our NICU, the statewide change in protocol for NBS of NICU patients led to an increase in false positive results and confirmatory testing at an added cost of ~$32,000 without an associated increase in true positive results or diagnosis of congenital disorders. The benefit of additional resource allocation for NBS in the NICU remains unclear.
Table: NBS results before and after practice change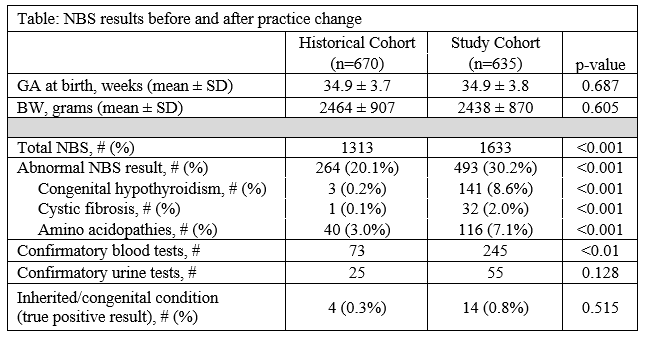
Objective: To assess the impact of change in the timing of NBS on the number of NBS performed, number of true and false positive test results, and associated cost of NBS.
Design/Methods: Retrospective cohort study performed at a level III NICU serving as a regional perinatal center for central and southern Illinois (ADC: 40, >700 annual admissions). Data was collected for inborn NICU admissions from two cohorts: 04/2018 – 04/2019 (historical cohort) and 05/2019 – 05/2020 (study cohort). Demographic data and NBS testing data were collected. Chi square was used for statistical analysis with p < 0.05 considered significant.
Results: 2946 NBS were performed on 1305 patients (median: two NBS/patient, range: one - four), including 1313 NBS performed on 670 patients in the historical cohort and 1633 NBS performed on 635 patients in the study cohort (table). Demographics data were similar between cohorts. Abnormal NBS were significantly more common after implementation of the practice change (20% vs 30%, OR: 1.72, 95% CI: 1.44-2.05, p < 0.001). Abnormal NBS results for congenital hypothyroidism, cystic fibrosis, and amino acidopathies were more frequent in the study cohort, but the incidence of abnormal NBS results for the other congenital conditions were similar. More follow-up testing/evaluation was required for the study vs historical cohort at an additional cost of ~$32,001 ($9,391 vs $26,392 for additional testing plus $15,000 for an addition 0.3 FTE for test tracking), but this was not associated with a statistically significant change in the number true positive NBS results ( < 1% for both cohorts).Conclusion(s): In our NICU, the statewide change in protocol for NBS of NICU patients led to an increase in false positive results and confirmatory testing at an added cost of ~$32,000 without an associated increase in true positive results or diagnosis of congenital disorders. The benefit of additional resource allocation for NBS in the NICU remains unclear.
Table: NBS results before and after practice change

