Pediatric Nutrition
Category: Abstract Submission
Pediatric Nutrition I
254 - Novel Central Line Securement Vest to Prevent Mechanical Complications of Tunneled Central Lines: Experience from a Cohort of Pediatric Patients with Intestinal Failure
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 254
Publication Number: 254.141
Publication Number: 254.141
Ryan St. Pierre-Hetz, UPMC Childrens Hospital of Pittsburgh, Pittsburgh, PA, United States; Kimberly Ackerman, UPMC Childrens Hospital of Pittsburgh, Pittsburgh, PA, United States; Christian P. Dresser, UPMC Childrens Hospital of Pittsburgh, Jefferson Hills, PA, United States; Jane Anne Yaworski, UPMC Childrens Hospital of Pittsburgh, Pittsburgh, PA, United States; Jeffrey A. Rudolph, UPMC Childrens Hospital of Pittsburgh, Pittsburgh, PA, United States; Stephen Wisniewski, University of Pittsburgh, Pittsburgh, PA, United States; Mioara Manole, University of Pittsburgh School of Medicine, Pittsburgh, PA, United States

Ryan St. Pierre-Hetz, MD (he/him/his)
Pediatric Emergency Medicine Fellow
UPMC Childrens Hospital of Pittsburgh
Pittsburgh, Pennsylvania, United States
Presenting Author(s)
Background: Tunneled central lines are used to deliver medications, hydration, and total parenteral nutrition. The current modality for their securement is by a transparent sterile adhesive. Mechanical line traumas, including line fissures, breaks, or dislodgements, occur frequently in children. A novel device, the Central Line Securement Vest, was created with the goal of protecting central lines from mechanical trauma.
Objective: We present here our experience with the Central Line Securement Vest and report its use in patients with intestinal failure treated at our institution.
Design/Methods: All patients who have used the Central Line Securement Vest at our institution during the last decade were identified. We reviewed the patients’ electronic records and compared the rate of line mechanical trauma, line infections, line replacements, ED visits, and hospital admissions for a period of 12 months before and after the use of the device.
Results: Ten patients were identified. Four patients had purchased the device at the time of line insertion. Six patients had a period of time of line use before starting using the device. The rate of line traumas and infections decreased after using the device: 0.19±0.15 vs. 0.05±0.04 trauma/month, pre- vs. post-device, p < 0.05. Similarly, the rate of line infections decreased post-device use: 0.18±0.13 vs. 0.09±0.06 infections/month, pre- vs. post-device, p < 0.05. The rate of line replacements, ED visits, and hospital admissions were similar pre- and post-device useConclusion(s): We report here our institution’s experience with a novel central line securement device designed to protect the line from mechanical trauma. Despite a limited number of subjects this research serves to introduce a completely novel pediatric medical device and illustrate potential benefits to vulnerable patients.
Conventional Central Line Securement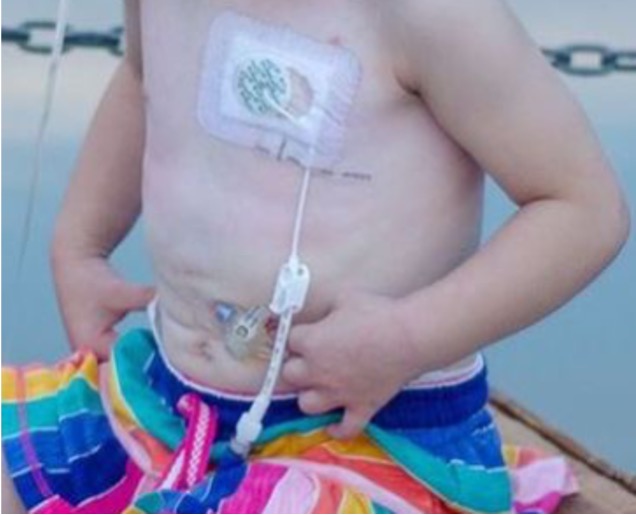 This figure shows the current method for the securement of central lines. The central line is protected solely by a clear adhesive dressing. Note that most of the central line is not protected, and is subjected to mechanical trauma: friction, torsion, bending, or traction, and to various bodily fluids or environmental agents.
This figure shows the current method for the securement of central lines. The central line is protected solely by a clear adhesive dressing. Note that most of the central line is not protected, and is subjected to mechanical trauma: friction, torsion, bending, or traction, and to various bodily fluids or environmental agents.
Central Line Securement Vest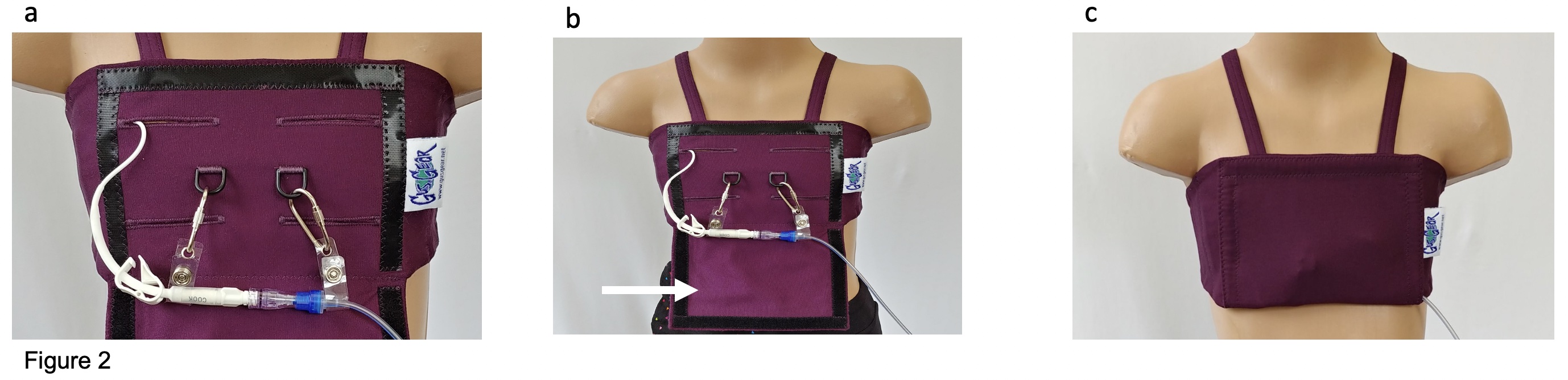 The central line is extruded through one of the four horizontal openings (a) and is secured using one fastener. If the line is connected to an infusion line, the second fastener is placed on the infusion line. The force applied to the infusion line is transmitted to the fasteners and does not exert tension on the central line. The protecting layer of the vest (b, white arrow) is closed using hook and loop tape and protects the entire central line from external contact (c). The infusion line is seen exiting the vest.
The central line is extruded through one of the four horizontal openings (a) and is secured using one fastener. If the line is connected to an infusion line, the second fastener is placed on the infusion line. The force applied to the infusion line is transmitted to the fasteners and does not exert tension on the central line. The protecting layer of the vest (b, white arrow) is closed using hook and loop tape and protects the entire central line from external contact (c). The infusion line is seen exiting the vest.
Objective: We present here our experience with the Central Line Securement Vest and report its use in patients with intestinal failure treated at our institution.
Design/Methods: All patients who have used the Central Line Securement Vest at our institution during the last decade were identified. We reviewed the patients’ electronic records and compared the rate of line mechanical trauma, line infections, line replacements, ED visits, and hospital admissions for a period of 12 months before and after the use of the device.
Results: Ten patients were identified. Four patients had purchased the device at the time of line insertion. Six patients had a period of time of line use before starting using the device. The rate of line traumas and infections decreased after using the device: 0.19±0.15 vs. 0.05±0.04 trauma/month, pre- vs. post-device, p < 0.05. Similarly, the rate of line infections decreased post-device use: 0.18±0.13 vs. 0.09±0.06 infections/month, pre- vs. post-device, p < 0.05. The rate of line replacements, ED visits, and hospital admissions were similar pre- and post-device useConclusion(s): We report here our institution’s experience with a novel central line securement device designed to protect the line from mechanical trauma. Despite a limited number of subjects this research serves to introduce a completely novel pediatric medical device and illustrate potential benefits to vulnerable patients.
Conventional Central Line Securement
 This figure shows the current method for the securement of central lines. The central line is protected solely by a clear adhesive dressing. Note that most of the central line is not protected, and is subjected to mechanical trauma: friction, torsion, bending, or traction, and to various bodily fluids or environmental agents.
This figure shows the current method for the securement of central lines. The central line is protected solely by a clear adhesive dressing. Note that most of the central line is not protected, and is subjected to mechanical trauma: friction, torsion, bending, or traction, and to various bodily fluids or environmental agents.Central Line Securement Vest
 The central line is extruded through one of the four horizontal openings (a) and is secured using one fastener. If the line is connected to an infusion line, the second fastener is placed on the infusion line. The force applied to the infusion line is transmitted to the fasteners and does not exert tension on the central line. The protecting layer of the vest (b, white arrow) is closed using hook and loop tape and protects the entire central line from external contact (c). The infusion line is seen exiting the vest.
The central line is extruded through one of the four horizontal openings (a) and is secured using one fastener. If the line is connected to an infusion line, the second fastener is placed on the infusion line. The force applied to the infusion line is transmitted to the fasteners and does not exert tension on the central line. The protecting layer of the vest (b, white arrow) is closed using hook and loop tape and protects the entire central line from external contact (c). The infusion line is seen exiting the vest.