Neonatal Fetal Nutrition & Metabolism
Category: Abstract Submission
Neonatal Fetal Nutrition & Metabolism I
282 - Trends in use of diazoxide before and after the introduction of oral dextrose gel for the treatment of neonatal hypoglycemia
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 282
Publication Number: 282.119
Publication Number: 282.119
Elizabeth Mancuso, John R. Oishei Children's Hospital, Buffalo, NY, United States; Mausma Bawa, Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo, Buffalo, NY, United States; Lucy D. Mastrandrea, Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo, Buffalo, NY, United States; Munmun Rawat, University at Buffalo, Buffalo, NY, United States; Praveen Chandrasekharan, Jacobs School of Medicine and Biomedical Sciences at the University at Buffalo, Buffalo, NY, United States
.jpeg.jpg)
Elizabeth Mancuso, MB BCh BAO
Pediatric Resident Physician
John R. Oishei Children's Hospital
Buffalo, New York, United States
Presenting Author(s)
Background: Diazoxide is used in the treatment of persistent hypoglycemia secondary to hyperinsulinism. A recently published retrospective cohort study involving 392 infants between 24-41 weeks' gestation admitted to neonatal intensive care units (NICUs) between 1997-2016, concluded that diazoxide use has increased over time. In our NICU, oral dextrose gel (ODG) was introduced in November of 2014 for treating asymptomatic hypoglycemia in infants ≥35 weeks, however, it is unclear if the introduction of ODG impacted use of diazoxide in this cohort.
Objective: To understand the characteristics of infants treated with diazoxide for neonatal hypoglycemia as well as to determine the impact of introduction of ODG therapy on diazoxide use in our NICU population between 2010-2020.
Design/Methods: A retrospective single-center study was conducted between 2010-2020 involving asymptomatic neonates ≥35 weeks admitted to the NICU at Children's Hospital of Buffalo. Total admission for hypoglycemia admitted to the NICU was collected. The trend in diazoxide use is represented as percentage of hypoglycemia admissions to the NICU. Data are mean ±SD or percent of cohort and represented based on parametric and non-parametric distribution. A Cochrane-Armitage trend analysis was performed to assess the effect of ODG on diazoxide use.
Results: In the time period queried, after introduction of ODG in the NBN, transfer of neonates ≥35 weeks to NICU for treatment of asymptomatic hypoglycemia decreased from 35 to 25/1000 live births. During this period, there were 6716 neonates diagnosed with hypoglycemia who required intravenous dextrose therapy. Diazoxide was used to treat hypoglycemia due to hyperinsulinemia in 74 infants who were mostly male and had birth weight < 3 kg (Fig. 1). There was no change in the trend of use of diazoxide before and after the introduction of ODG therapy for treatment of asymptomatic neonatal hypoglycemia (Figure 2). Most hyperinsulinemic neonates requiring diazoxide received 0 (82%) or 1 dose of ODG (7%) and subsequently required glucose infusion rates >10 mg/kg/min. Pulmonary hypertension, a recognized side effect of diazoxide, was not reported in any of these neonates.Conclusion(s): In neonates ≥35 weeks with hypoglycemia, the introduction of ODG did not impact the use of diazoxide. There were no long-term adverse events other than a minority of neonates having hypertrichosis in our cohort, reported on endocrine follow-up. Our data would be useful for neonatologists and endocrinologists to understand the characteristics of neonates with hyperinsulinemic hypoglycemia.
EMancuso CV.pdf
Figure 2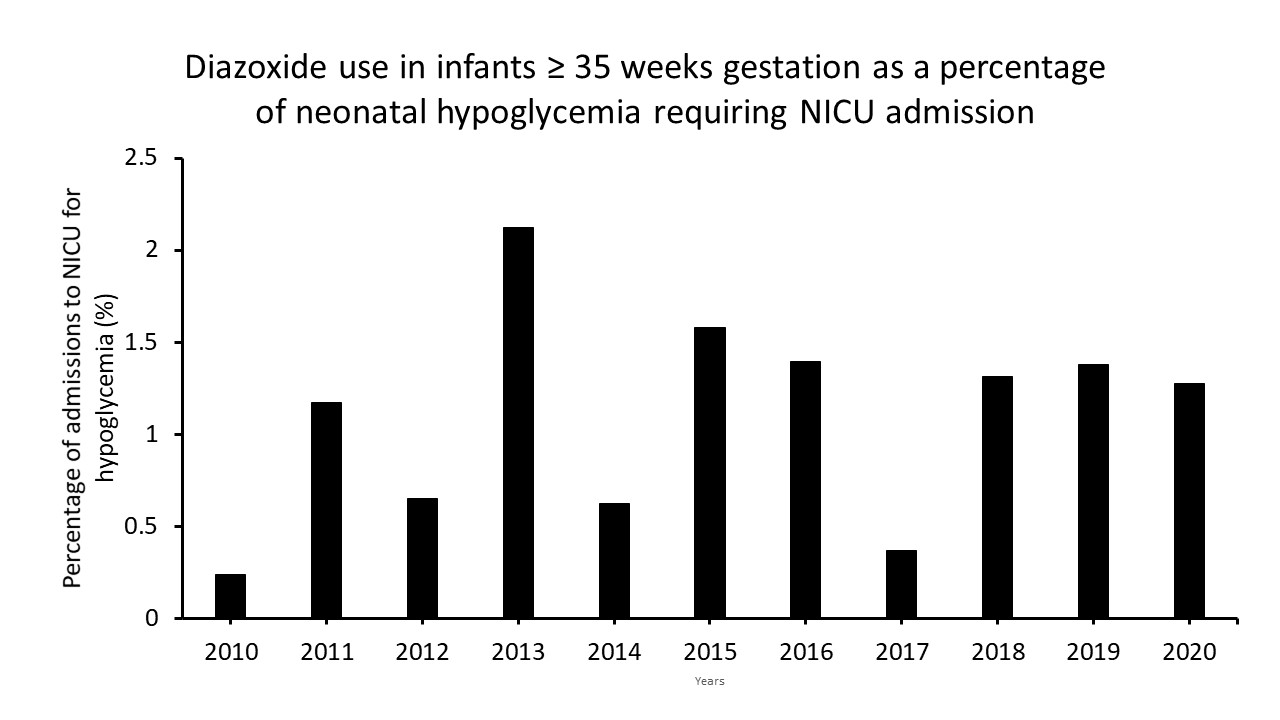
Objective: To understand the characteristics of infants treated with diazoxide for neonatal hypoglycemia as well as to determine the impact of introduction of ODG therapy on diazoxide use in our NICU population between 2010-2020.
Design/Methods: A retrospective single-center study was conducted between 2010-2020 involving asymptomatic neonates ≥35 weeks admitted to the NICU at Children's Hospital of Buffalo. Total admission for hypoglycemia admitted to the NICU was collected. The trend in diazoxide use is represented as percentage of hypoglycemia admissions to the NICU. Data are mean ±SD or percent of cohort and represented based on parametric and non-parametric distribution. A Cochrane-Armitage trend analysis was performed to assess the effect of ODG on diazoxide use.
Results: In the time period queried, after introduction of ODG in the NBN, transfer of neonates ≥35 weeks to NICU for treatment of asymptomatic hypoglycemia decreased from 35 to 25/1000 live births. During this period, there were 6716 neonates diagnosed with hypoglycemia who required intravenous dextrose therapy. Diazoxide was used to treat hypoglycemia due to hyperinsulinemia in 74 infants who were mostly male and had birth weight < 3 kg (Fig. 1). There was no change in the trend of use of diazoxide before and after the introduction of ODG therapy for treatment of asymptomatic neonatal hypoglycemia (Figure 2). Most hyperinsulinemic neonates requiring diazoxide received 0 (82%) or 1 dose of ODG (7%) and subsequently required glucose infusion rates >10 mg/kg/min. Pulmonary hypertension, a recognized side effect of diazoxide, was not reported in any of these neonates.Conclusion(s): In neonates ≥35 weeks with hypoglycemia, the introduction of ODG did not impact the use of diazoxide. There were no long-term adverse events other than a minority of neonates having hypertrichosis in our cohort, reported on endocrine follow-up. Our data would be useful for neonatologists and endocrinologists to understand the characteristics of neonates with hyperinsulinemic hypoglycemia.
EMancuso CV.pdf
Figure 2

