Global Neonatal & Children's Health
Category: Abstract Submission
Global Child and Adolescent Health I
147 - Validation of a Low-Cost Pulse Oximeter for Oxygen Saturation Measurement in Neonates
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 147
Nora Switchenko, University of Alabama at Birmingham, Homewood, AL, United States; Jyoti Lakhwani, University Teaching Hospital Zambia, Lusaka, Lusaka, Zambia; Albert Manasyan, University of Alabama School of Medicine, Birmingham, AL, United States; AKM F. Rahman, University of Alabama at Birmingham, Vestavia, AL, United States; Waldemar A. Carlo, University of Alabama at Birmingham, Birmingham, AL, United States; Kunda Kapembwa, University Teaching Hospital, Women and Newborn Hospital, Lusaka, Lusaka, Zambia

Nora Switchenko, MD
Assistant Professor
University of Alabama at Birmingham
Homewood, Alabama, United States
Presenting Author(s)
Background: Detection of low oxygen levels in neonates is essential as hypoxemia may be the only sign of an underlying respiratory or cardiac disease process. Pulse oximetry is a non-invasive and easy to administer clinical tool used to measure oxygen saturation (SpO2), but due to high cost, pulse oximetry is not available in many low-resource settings. Validation of a low-cost pulse oximeter for use in newborns would increase healthcare system capacity for recognition and treatment of hypoxemia in low-resource settings.
Objective: To determine the accuracy of a low-cost pulse oximeter in measuring SpO2 in neonates.
Design/Methods: This validation study compared measurements from a low-cost pulse oximeter designed for infants, the Yonker K1 model (Xuzhou Yongkang Electronic Technology Corp, Xuzhou, China, $40), to those from a high-cost pulse oximeter, the 5v Masimo model (Masimo Corp, Irvine, CA, USA, $1100). SpO2 measurements were made on neonates cared for in the well-baby nurseries (postnatal care and the kangaroo mother care wards) at the University Teaching Hospital in Lusaka, Zambia. Measurements were made simultaneously with each device on the right hand (preductal) and either foot (postductal). A Bland Altman plot assessed agreement in measurements made by the low and high cost devices. The predetermined maximum allowed difference between measurements was ± 3.
Results: SpO2 was measured on 419 neonates (Figure 1). The mean gestational age was 36.9 weeks (SD 3.4) and the mean birthweight was 2.8 kg (SD 0.8 kg). The mean preductal Sp02 measured was 94.9 (SD 3.2) and 94.5 (SD 3.7) by the Masimo and Yonker devices respectively. The mean postductal Sp02 measured was 94.9 (SD 3.2) and 94.4 (SD 3.9) by the Masimo and Yonker devices respectively. The limits of agreement (determined by ±2SD) are larger than the predetermined maximum allowed difference for both preductal and postductal Spo2 measurements (Figures 2 and 3). 91% (preductal) and 90% (postductal) of the Sp02 measurements by the Yonker device are within ± 3 points from the SpO2 measurements by the Masimo device.Conclusion(s): Measurement of SpO2 by this low cost infant pulse oximeter was not accurate within a maximum allowed difference of ± 3. Validation of low-cost pulse oximeters is needed to ensure acceptable performance and quality.
Figure 1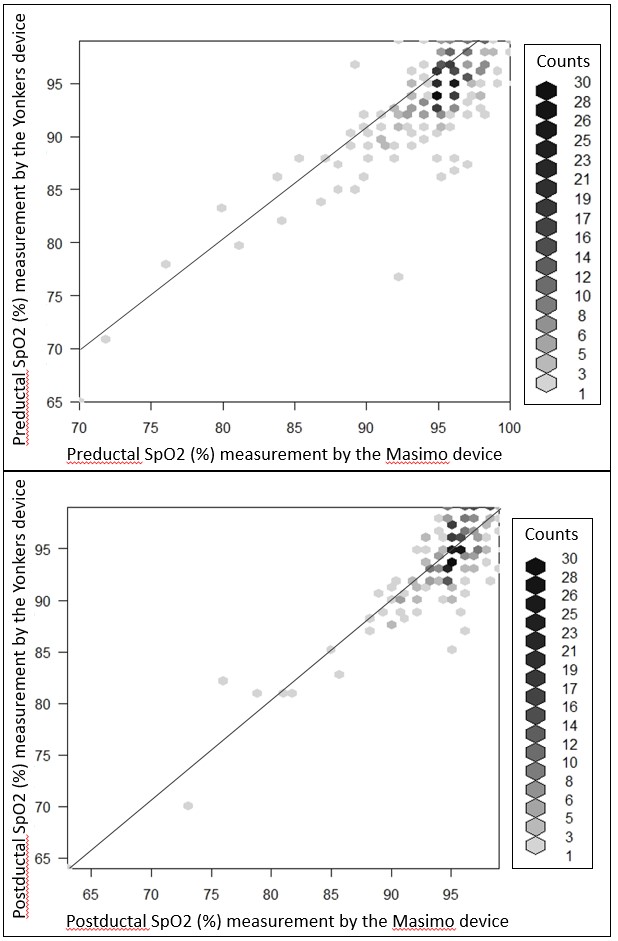 Scatter plots of Sp02 measurements with Masimo and Yonker devices. Points on the graph may represent more than one data point indicated by shading as described by the counts listed to the right of the graph. The line of identity is shown in both graphs.
Scatter plots of Sp02 measurements with Masimo and Yonker devices. Points on the graph may represent more than one data point indicated by shading as described by the counts listed to the right of the graph. The line of identity is shown in both graphs.
Figure 2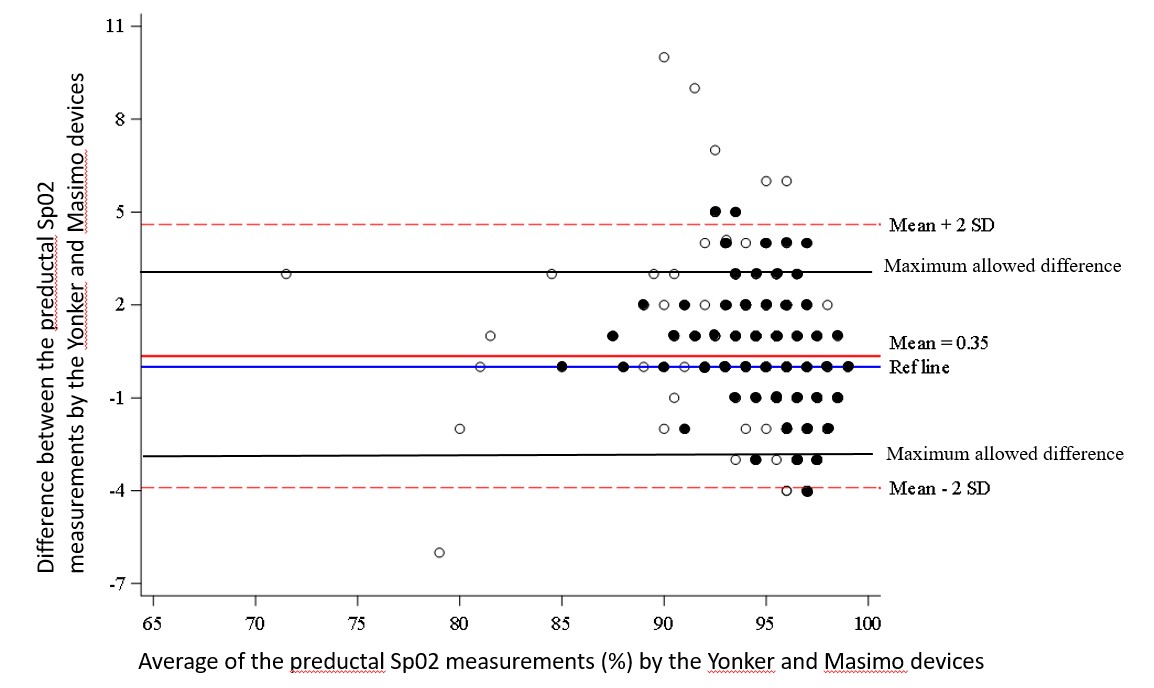 Bland Altman plot for the preductal SpO2 measurements by the Yonker and Masimo devices The limits of agreement (determined by ±2SD) are larger than the predetermined maximum allowed difference for the preductal Spo2 measurements. Dark circles represent more than one data point.
Bland Altman plot for the preductal SpO2 measurements by the Yonker and Masimo devices The limits of agreement (determined by ±2SD) are larger than the predetermined maximum allowed difference for the preductal Spo2 measurements. Dark circles represent more than one data point.
Objective: To determine the accuracy of a low-cost pulse oximeter in measuring SpO2 in neonates.
Design/Methods: This validation study compared measurements from a low-cost pulse oximeter designed for infants, the Yonker K1 model (Xuzhou Yongkang Electronic Technology Corp, Xuzhou, China, $40), to those from a high-cost pulse oximeter, the 5v Masimo model (Masimo Corp, Irvine, CA, USA, $1100). SpO2 measurements were made on neonates cared for in the well-baby nurseries (postnatal care and the kangaroo mother care wards) at the University Teaching Hospital in Lusaka, Zambia. Measurements were made simultaneously with each device on the right hand (preductal) and either foot (postductal). A Bland Altman plot assessed agreement in measurements made by the low and high cost devices. The predetermined maximum allowed difference between measurements was ± 3.
Results: SpO2 was measured on 419 neonates (Figure 1). The mean gestational age was 36.9 weeks (SD 3.4) and the mean birthweight was 2.8 kg (SD 0.8 kg). The mean preductal Sp02 measured was 94.9 (SD 3.2) and 94.5 (SD 3.7) by the Masimo and Yonker devices respectively. The mean postductal Sp02 measured was 94.9 (SD 3.2) and 94.4 (SD 3.9) by the Masimo and Yonker devices respectively. The limits of agreement (determined by ±2SD) are larger than the predetermined maximum allowed difference for both preductal and postductal Spo2 measurements (Figures 2 and 3). 91% (preductal) and 90% (postductal) of the Sp02 measurements by the Yonker device are within ± 3 points from the SpO2 measurements by the Masimo device.Conclusion(s): Measurement of SpO2 by this low cost infant pulse oximeter was not accurate within a maximum allowed difference of ± 3. Validation of low-cost pulse oximeters is needed to ensure acceptable performance and quality.
Figure 1
 Scatter plots of Sp02 measurements with Masimo and Yonker devices. Points on the graph may represent more than one data point indicated by shading as described by the counts listed to the right of the graph. The line of identity is shown in both graphs.
Scatter plots of Sp02 measurements with Masimo and Yonker devices. Points on the graph may represent more than one data point indicated by shading as described by the counts listed to the right of the graph. The line of identity is shown in both graphs.Figure 2
 Bland Altman plot for the preductal SpO2 measurements by the Yonker and Masimo devices The limits of agreement (determined by ±2SD) are larger than the predetermined maximum allowed difference for the preductal Spo2 measurements. Dark circles represent more than one data point.
Bland Altman plot for the preductal SpO2 measurements by the Yonker and Masimo devices The limits of agreement (determined by ±2SD) are larger than the predetermined maximum allowed difference for the preductal Spo2 measurements. Dark circles represent more than one data point.