Neonatal Infectious Diseases/Immunology
Category: Abstract Submission
Neonatal Infectious Diseases/Immunology: COVID-19
278 - BOOSTING THE MATERNAL ANTIBODY RESPONSE AGAINST SARS-CoV-2 RESULTS IN INCREASED TRANSFER TO INFANTS DURING PREGNANCY FETAL IMMUNE RESPONSE TO SARS-CoV-2 INFECTION AND IMMUNIZATION DURING PREGNANCY
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 278
Publication Number: 278.226
Publication Number: 278.226
Kristen Smith, National Capital Consortium, Bethesda, MD, United States; Zachary Weber, Walter Reed Military Medical Center, Silver Spring, MD, United States; Krista M. Mehlhaff, Uniformed Services University of the Health Sciences F. Edward Hebert School of Medicine, Bethesda, MD, United States; Eric Laing, Uniformed Services University of the Health Sciences F. Edward Hebert School of Medicine, Bethedsa, MD, United States; Amanda Cain, Walter Reed National Military Medical Center, Washington, DC, United States; Allison M. Malloy, USUHS, Bethesda, MD, United States
- KS
Kristen Smith, MD
Neonatal-Perinatal Fellow
Walter Reed National Military Medical Center
Bethesda, Maryland, United States
Presenting Author(s)
Background: Passive transfer of IgG antibodies through the placenta during pregnancy, primarily third trimester, reduces the incidence of various infectious diseases in infants. Increased maternal antibody levels to influenza, respiratory syncytial virus, pertussis and rubella as well as implementation of third trimester influenza and pertussis vaccination strategies correlate with improved infant protection against these infections. The development of protective immunity against SARS-CoV-2 and the response to novel messenger RNA vaccines in pregnant women is currently poorly understood and limits the ability to determine whether passive immunity during pregnancy can protect infants.
Objective: Evaluate the passive transfer of antibodies during pregnancy after SARS-CoV-2 infection or maternal immunization.
Design/Methods: Prospective multi-center cohort study of pregnant women ≥18 years of age who acquired SARS-CoV-2 infection and/or received at least one dose of mRNA SARS-CoV-2 vaccine before or during pregnancy. Native infection confirmation was evaluated using serum nucleoprotein concentration, which is not found in infection naïve immunized patients. Fluorescently stained antibodies against the spike protein of SARS-CoV-2 from maternal serum and cord blood were quantitatively measured using mean fluorescence intensity (MFI) and compared by vaccination status using exact Wilcoxon rank sum tests.
Results: Of the eight couplets with available cord blood, each mother was infected with SARS-CoV-2 prior to delivery with confirmatory nucleoprotein concentrations in both maternal and fetal circulation. Three of these mothers were vaccinated prior to delivery. Quantitative measurement and vaccination status are summarized (Figure). Antibody concentrations, as measured by MFI, were higher for mothers who were infected and vaccinated prior to delivery, versus those who were unimmunized, in both maternal (p = 0.04) and fetal circulation (p = 0.04).Conclusion(s): Our findings demonstrate that native infection followed by immunization resulted in a higher antibody titer in a small cohort of mothers and their infants, respectively. Our results suggest that priming of the maternal immune system followed by boosting with immunization is required to induce a higher magnitude of SARS-CoV-2 specific antibodies in mothers resulting in increased passive transfer during pregnancy to their infants.
Figure.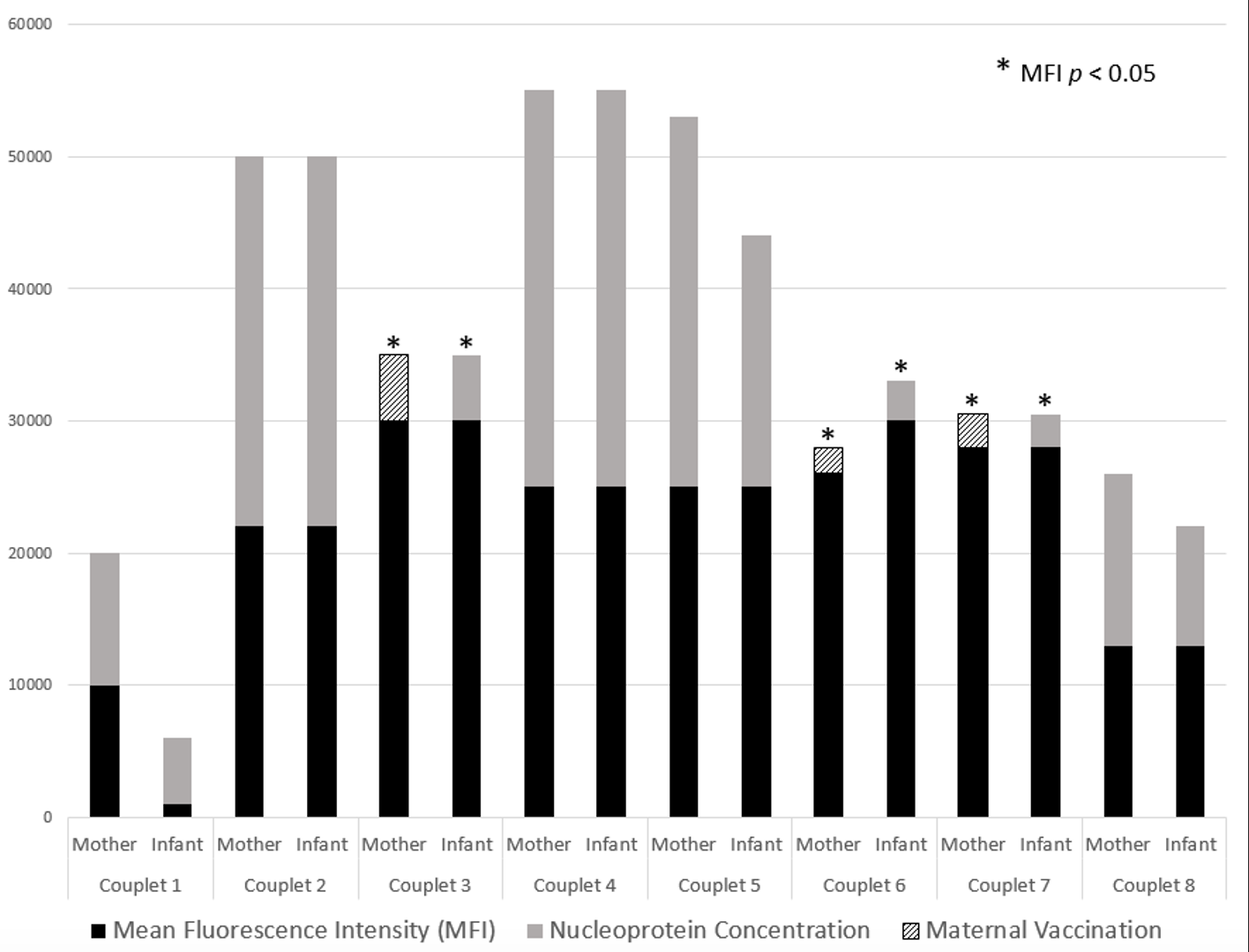 SARS-CoV-2 IgG concentrations present in maternal and fetal circulation represented by mean fluorescence intensity (MFI). Native maternal infection confirmed via SARS-CoV-2 nucleoprotein presence in maternal serum and infant cord blood. A statistically significant association was found in the MFI of mothers who experienced both infection and immunization during their pregnancy (p = 0.04) and this correlated with a statistically significant MFI in their respective offspring (p = 0.04). There was no statistical significance in unimmunized, but previously infected, mothers or their offspring.
SARS-CoV-2 IgG concentrations present in maternal and fetal circulation represented by mean fluorescence intensity (MFI). Native maternal infection confirmed via SARS-CoV-2 nucleoprotein presence in maternal serum and infant cord blood. A statistically significant association was found in the MFI of mothers who experienced both infection and immunization during their pregnancy (p = 0.04) and this correlated with a statistically significant MFI in their respective offspring (p = 0.04). There was no statistical significance in unimmunized, but previously infected, mothers or their offspring.
Objective: Evaluate the passive transfer of antibodies during pregnancy after SARS-CoV-2 infection or maternal immunization.
Design/Methods: Prospective multi-center cohort study of pregnant women ≥18 years of age who acquired SARS-CoV-2 infection and/or received at least one dose of mRNA SARS-CoV-2 vaccine before or during pregnancy. Native infection confirmation was evaluated using serum nucleoprotein concentration, which is not found in infection naïve immunized patients. Fluorescently stained antibodies against the spike protein of SARS-CoV-2 from maternal serum and cord blood were quantitatively measured using mean fluorescence intensity (MFI) and compared by vaccination status using exact Wilcoxon rank sum tests.
Results: Of the eight couplets with available cord blood, each mother was infected with SARS-CoV-2 prior to delivery with confirmatory nucleoprotein concentrations in both maternal and fetal circulation. Three of these mothers were vaccinated prior to delivery. Quantitative measurement and vaccination status are summarized (Figure). Antibody concentrations, as measured by MFI, were higher for mothers who were infected and vaccinated prior to delivery, versus those who were unimmunized, in both maternal (p = 0.04) and fetal circulation (p = 0.04).Conclusion(s): Our findings demonstrate that native infection followed by immunization resulted in a higher antibody titer in a small cohort of mothers and their infants, respectively. Our results suggest that priming of the maternal immune system followed by boosting with immunization is required to induce a higher magnitude of SARS-CoV-2 specific antibodies in mothers resulting in increased passive transfer during pregnancy to their infants.
Figure.
 SARS-CoV-2 IgG concentrations present in maternal and fetal circulation represented by mean fluorescence intensity (MFI). Native maternal infection confirmed via SARS-CoV-2 nucleoprotein presence in maternal serum and infant cord blood. A statistically significant association was found in the MFI of mothers who experienced both infection and immunization during their pregnancy (p = 0.04) and this correlated with a statistically significant MFI in their respective offspring (p = 0.04). There was no statistical significance in unimmunized, but previously infected, mothers or their offspring.
SARS-CoV-2 IgG concentrations present in maternal and fetal circulation represented by mean fluorescence intensity (MFI). Native maternal infection confirmed via SARS-CoV-2 nucleoprotein presence in maternal serum and infant cord blood. A statistically significant association was found in the MFI of mothers who experienced both infection and immunization during their pregnancy (p = 0.04) and this correlated with a statistically significant MFI in their respective offspring (p = 0.04). There was no statistical significance in unimmunized, but previously infected, mothers or their offspring.