Allergy, Immunology and Rheumatology
Category: Abstract Submission
Allergy, Immunology, and Rheumatology II
622 - Burden of Atopic Dermatitis in Infants/Preschoolers in the EPI-CARE International Survey
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 622
Stephan Weidinger, University Hospital Schleswig-Holstein, Kiel, Schleswig-Holstein, Germany; Eric L. Simpson, Oregon Health & Science University, Portland, OR, United States; Jonathan I. Silverberg, George Washington University School of Medicine, Baltimore, MD, United States; Ana B. Rossi, Sanofi-Aventis, Cambridge, MA, United States; Lysel Brignoli, Cerner Enviza, Paris, Ile-de-France, France; Ryan B. Thomas, Regeneron, Montclair, NJ, United States; Gaelle Bego-Le-Bagousse, Sanofi, Chilly-Mazarin, Ile-de-France, France; Ashish Bansal, Regeneron pharmaceuticals, Tarrytown, NY, United States; Chien-chia Chuang, Sanofi, Cambridge, MA, United States

Deborah Griffis, PharmD
Associate Director Field Medical, Dermatology
Regeneron
Denver, Colorado, United States
Presenting Author(s)
Background: The global burden of atopic dermatitis (AD) in infants/preschoolers is not well understood. Improved knowledge may be informative for disease management strategies.
Objective: Here, we report on AD severity and outcomes in children aged 6 months to < 6 years in the EPI-CARE cross-sectional, web-based survey.
Design/Methods: Eligible children < 6 years had “diagnosed AD”, i.e. met all International Study of Asthma and Allergies in Childhood criteria, had a parent/caregiver-report of physician-diagnosed eczema, and itchy rash affecting, at any time, the face (cheeks, forehead) and elbows to wrist or knee to ankle. AD severity was assessed using Patient Global Assessment, categorized over the last week by parents/caregivers as mild, moderate, or severe. Outcomes were stratified by geographic region/AD severity including atopic comorbidities, worst itch/worst skin pain/overall sleep disturbance in the past 24 hours, eczema-related hospitalization, flares in the past month, health-related quality of life (Children's Dermatology Life Quality Index [CDLQI] or Infants’ Dermatitis Quality of Life Index [IDQOL]), and missed school days in the past 4 weeks (4 to < 6 year-olds).
Results: The mean (standard deviation [SD]) age of the 1,489 participants was 3.0 (1.6) years. The majority (61.6%) had mild AD, and ≥1 atopic comorbidity was reported in 88.3%, 92.1%, and 95.8% of patients with mild, moderate, and severe AD, respectively (Table). Infants/preschoolers with moderate/severe AD had worse itch, skin pain, and sleep disturbances over the past 24 hours than those with mild AD (Table). 54.1% of patients with severe AD were hospitalized in the past 12 months, compared with 35.0% with moderate and 32.1% with mild AD (Table). In total, 50.6%, 18.6%, and 6.3% of patients with severe, moderate, and mild AD had >2 flares in the past month, and 50.7% of patients with severe AD had an average flare duration of ≥2 weeks (moderate AD: 20.8%; mild AD: 10.0%) (Table). Higher CDLQI/IDQOL mean scores were seen with increasing AD severity (Table). The majority (78.3%) of preschoolers missed at least 1 school day in the past 4 weeks, with a mean (SD) of 5.1 (5.7), 7.3 (7.1) and 12.1 (7.8) days lost in patients with mild, moderate, and severe AD, respectively.Conclusion(s): Infants/preschoolers with mild to severe AD experience a substantial disease burden across multiple domains, with a trend of increased burden with more severe AD.
Table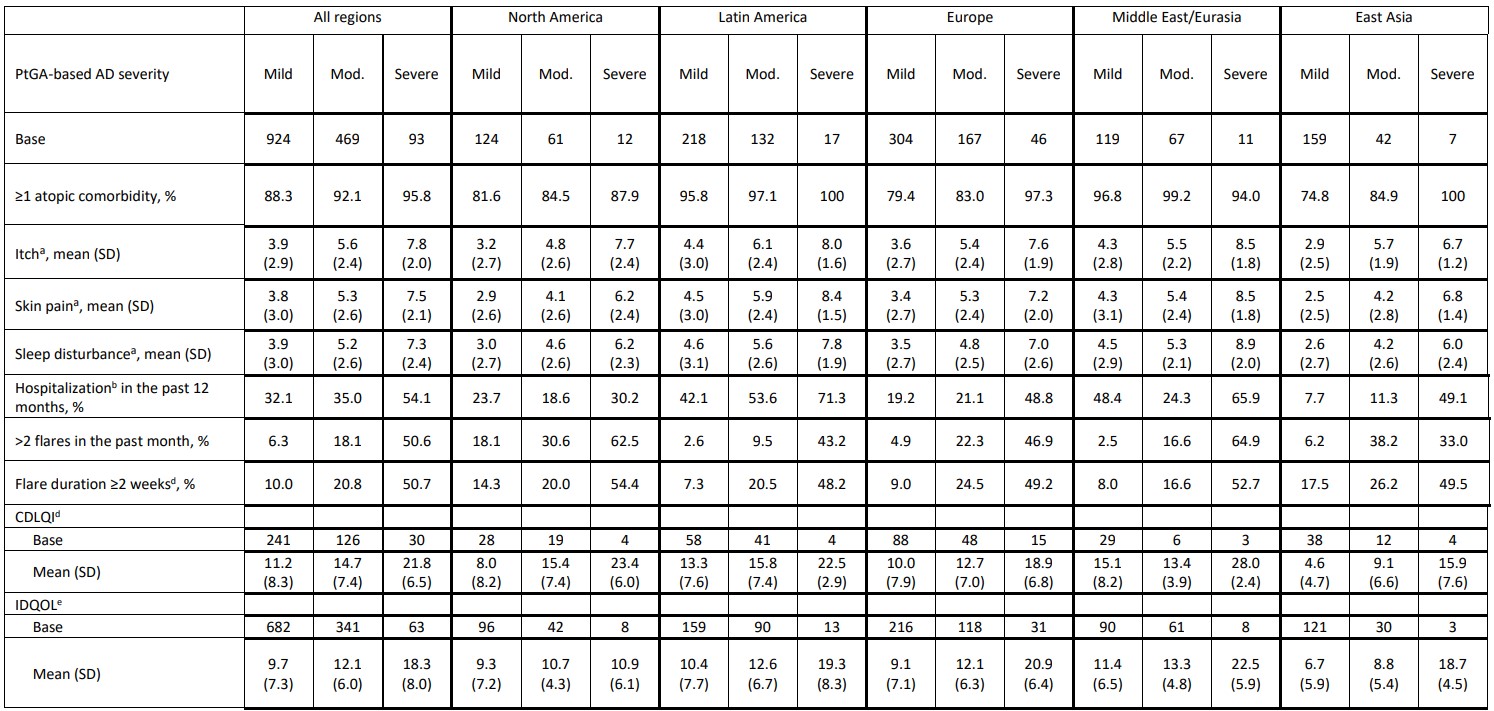 aWorst instance as assessed by Numeric Rating Scale; 0–10 higher scores indicate worse severity
aWorst instance as assessed by Numeric Rating Scale; 0–10 higher scores indicate worse severity
bHospitalization due to eczema
cAverage duration of flares in the past month
dCDLQI was administered in preschoolers aged 4 to < 6 years if parents/guardians agreed to pass control to their child. Range 0–30, with higher scores indicating greater negative impact
eIDQOL was administered to parents/guardians of infants/preschoolers aged < 4 years old and those aged 4 to < 6 years old for whom parents/guardians did not agree to pass control. Range 0–30, with higher scores indicating greater negative impact
Objective: Here, we report on AD severity and outcomes in children aged 6 months to < 6 years in the EPI-CARE cross-sectional, web-based survey.
Design/Methods: Eligible children < 6 years had “diagnosed AD”, i.e. met all International Study of Asthma and Allergies in Childhood criteria, had a parent/caregiver-report of physician-diagnosed eczema, and itchy rash affecting, at any time, the face (cheeks, forehead) and elbows to wrist or knee to ankle. AD severity was assessed using Patient Global Assessment, categorized over the last week by parents/caregivers as mild, moderate, or severe. Outcomes were stratified by geographic region/AD severity including atopic comorbidities, worst itch/worst skin pain/overall sleep disturbance in the past 24 hours, eczema-related hospitalization, flares in the past month, health-related quality of life (Children's Dermatology Life Quality Index [CDLQI] or Infants’ Dermatitis Quality of Life Index [IDQOL]), and missed school days in the past 4 weeks (4 to < 6 year-olds).
Results: The mean (standard deviation [SD]) age of the 1,489 participants was 3.0 (1.6) years. The majority (61.6%) had mild AD, and ≥1 atopic comorbidity was reported in 88.3%, 92.1%, and 95.8% of patients with mild, moderate, and severe AD, respectively (Table). Infants/preschoolers with moderate/severe AD had worse itch, skin pain, and sleep disturbances over the past 24 hours than those with mild AD (Table). 54.1% of patients with severe AD were hospitalized in the past 12 months, compared with 35.0% with moderate and 32.1% with mild AD (Table). In total, 50.6%, 18.6%, and 6.3% of patients with severe, moderate, and mild AD had >2 flares in the past month, and 50.7% of patients with severe AD had an average flare duration of ≥2 weeks (moderate AD: 20.8%; mild AD: 10.0%) (Table). Higher CDLQI/IDQOL mean scores were seen with increasing AD severity (Table). The majority (78.3%) of preschoolers missed at least 1 school day in the past 4 weeks, with a mean (SD) of 5.1 (5.7), 7.3 (7.1) and 12.1 (7.8) days lost in patients with mild, moderate, and severe AD, respectively.Conclusion(s): Infants/preschoolers with mild to severe AD experience a substantial disease burden across multiple domains, with a trend of increased burden with more severe AD.
Table
 aWorst instance as assessed by Numeric Rating Scale; 0–10 higher scores indicate worse severity
aWorst instance as assessed by Numeric Rating Scale; 0–10 higher scores indicate worse severitybHospitalization due to eczema
cAverage duration of flares in the past month
dCDLQI was administered in preschoolers aged 4 to < 6 years if parents/guardians agreed to pass control to their child. Range 0–30, with higher scores indicating greater negative impact
eIDQOL was administered to parents/guardians of infants/preschoolers aged < 4 years old and those aged 4 to < 6 years old for whom parents/guardians did not agree to pass control. Range 0–30, with higher scores indicating greater negative impact
