Gastroenterology/Hepatology
Category: Abstract Submission
Gastroenterology/Hepatology I
6 - Identification and Testing in Infants With Perinatal Hepatitis C Exposure
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 6
Publication Number: 6.207
Publication Number: 6.207
Natalie Morris, Cooper Medical School of Rowan University, Philadelphia, PA, United States; Krystal Hunter, Cooper Medical School of Rowan University, Camden, NJ, United States; Vishwanath Bhat, The Children's Regional Hospital at Cooper, Camden, NJ, United States; Alla Kushnir, The Children's Regional Hospital at Cooper, Camden, NJ, United States

Natalie Morris
Medical Student
Cooper Medical School of Rowan University
Philadelphia, Pennsylvania, United States
Presenting Author(s)
Background: The incidence of hepatitis C virus (HCV) infection has seen a rising trend in recent years, primarily driven by injection drug use. In women of childbearing age, infection rates doubled from 2006 to 2014. As a blood-borne disease, HCV carries a risk for vertical transmission. Infants born to women infected with HCV tend to be smaller for their gestational age and require Neonatal Intensive Care Unit (NICU) admission and ventilation more often than their counterparts. The North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition (NASPGHAN) recommends testing children born to HCV infected mothers at 18 months with anti-HCV antibody testing or serum HCV RNA after two months of age. Studies suggest that exposed infants are not being adequately tested for HCV.
Objective: To determine whether infants exposed to HCV in utero had prenatal HCV exposure documented in electronic health records (EHR) and if they were adequately tested for HCV after birth.
Design/Methods: A retrospective review of infants born in a tertiary urban hospital in NJ from 1/1/2011 to 1/1/2021. Records of infants born to mothers with prenatal diagnosis of HCV were examined to determine whether they were documented to have HCV exposure in EHR. Infants with perinatal HCV exposure were evaluated to determine if anti-HCV antibody testing at 18 months or serum HCV RNA after two months of age was performed. Infants who received the exposure in EHR but were lost to follow-up prior to two years of age and not tested in that time period were excluded. General maternal and neonatal clinical and demographic data was analyzed.
Results: Of the 142 mothers with prenatal diagnosis of HCV, 114 (80%) infants had a diagnosis of HCV exposure in EHR. Of the 77 infants with follow up data available by 24 months of age, 60 (78%) were tested with 41 out of 77 (53%) receiving adequate testing. Of infants documented as HCV exposed, 94.5% of mothers were Caucasian, 98% of mothers had a history of opioid use, 90.3% had opioid use during pregnancy, and 3.5% of mothers were diagnosed at delivery.Conclusion(s): A significant proportion of infants born to HCV-infected mothers were either not identified at birth (20%) or did not receive appropriate testing on follow-up (47%). Further work needs to be done to improve documentation of HCV exposure at birth and follow-up testing in order to avoid missing congenitally acquired HCV.
Infants Born to HCV Positive Mothers.png) The number of infants born per year at a tertiary urban NJ hospital to HCV positive mothers.
The number of infants born per year at a tertiary urban NJ hospital to HCV positive mothers.
Percent of Infants Born to Hepatitis C Mothers with Adequate Testing by 2 Years of Age of Infants Tested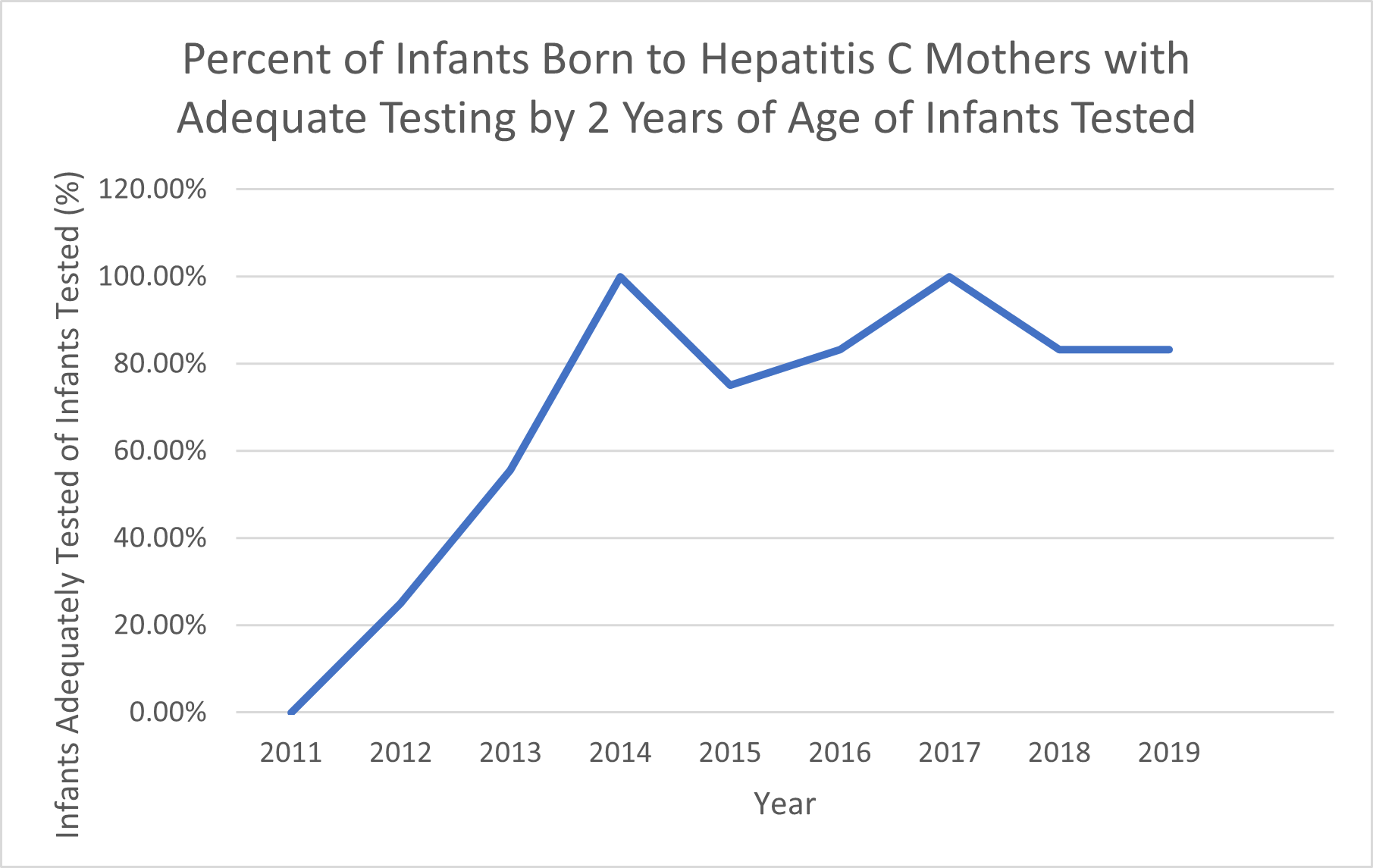 The percentage of infants born to HCV positive mothers that received adequate testing of infants that received any HCV testing. Adequate testing defined by NASPGHAN.
The percentage of infants born to HCV positive mothers that received adequate testing of infants that received any HCV testing. Adequate testing defined by NASPGHAN.
Objective: To determine whether infants exposed to HCV in utero had prenatal HCV exposure documented in electronic health records (EHR) and if they were adequately tested for HCV after birth.
Design/Methods: A retrospective review of infants born in a tertiary urban hospital in NJ from 1/1/2011 to 1/1/2021. Records of infants born to mothers with prenatal diagnosis of HCV were examined to determine whether they were documented to have HCV exposure in EHR. Infants with perinatal HCV exposure were evaluated to determine if anti-HCV antibody testing at 18 months or serum HCV RNA after two months of age was performed. Infants who received the exposure in EHR but were lost to follow-up prior to two years of age and not tested in that time period were excluded. General maternal and neonatal clinical and demographic data was analyzed.
Results: Of the 142 mothers with prenatal diagnosis of HCV, 114 (80%) infants had a diagnosis of HCV exposure in EHR. Of the 77 infants with follow up data available by 24 months of age, 60 (78%) were tested with 41 out of 77 (53%) receiving adequate testing. Of infants documented as HCV exposed, 94.5% of mothers were Caucasian, 98% of mothers had a history of opioid use, 90.3% had opioid use during pregnancy, and 3.5% of mothers were diagnosed at delivery.Conclusion(s): A significant proportion of infants born to HCV-infected mothers were either not identified at birth (20%) or did not receive appropriate testing on follow-up (47%). Further work needs to be done to improve documentation of HCV exposure at birth and follow-up testing in order to avoid missing congenitally acquired HCV.
Infants Born to HCV Positive Mothers
.png) The number of infants born per year at a tertiary urban NJ hospital to HCV positive mothers.
The number of infants born per year at a tertiary urban NJ hospital to HCV positive mothers.Percent of Infants Born to Hepatitis C Mothers with Adequate Testing by 2 Years of Age of Infants Tested
 The percentage of infants born to HCV positive mothers that received adequate testing of infants that received any HCV testing. Adequate testing defined by NASPGHAN.
The percentage of infants born to HCV positive mothers that received adequate testing of infants that received any HCV testing. Adequate testing defined by NASPGHAN.