Emergency Medicine: All Areas
Category: Abstract Submission
Emergency Medicine V
339 - Implementation Of A Bronchiolitis Scoring System Within The Pediatric Emergency Department
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 339
Publication Number: 339.204
Publication Number: 339.204
Sarah B. Bingham, University of Alabama School of Medicine, Birmingham, AL, United States; Michael S. Mitchell, Wake Forest School of Medicine of Wake Forest Baptist Medical Center, Winston Salem, NC, United States; David M. Cline, Wake Forest Baptist Health - Brenner Children's Hospital, Winston-Salem, NC, United States

Sarah B. Bingham, MD
Assistant Professor
University of Alabama School of Medicine
Birmingham, Alabama, United States
Presenting Author(s)
Background: High flow nasal cannula (HFNC) is a generally well-tolerated non-invasive ventilation therapy utilized in the management of bronchiolitis. Although research has been limited to its use in the intensive care units, there has been growing support for protocol development within Emergency Departments. We aimed to standardize the implementation of HFNC within our pediatric emergency department based upon illness severity.
Objective: The aim of this study was to assess protocol implementation with throughput and quality metrics to include time to HFNC, ED length of stay (LOS), hospital LOS and rate of escalation to a higher level of service.
Design/Methods: We performed a retrospective chart review of patients under age 24 months admitted with bronchiolitis as their primary diagnosis. The data was collected November 2018-April 2019 (pre-implementation) and November 2019-April 2020 (post-implementation). The primary outcome measures included ED LOS, time to implementation of HFNC, hospital LOS and rate of escalation to a higher level of service. Secondary outcome measures included utilization of bronchodilators, antibiotics and steroids. Viral panel and chest radiograph acquisition and results were also correlated with bronchiolitis severity. Chi square test and Wilcoxon Rank Sum procedure with Kruskal-Wallis test were used to compare groups as appropriate.
Results: A total of 353 patients were included in this study, 180 in the pre-implementation group and 173 in the post-implementation group. HFNC was started in 25 patients in the pre-implementation group compared to 28 in the post-implementation (13.9% vs 16.2% respectively). The mean time to initiation of HFNC was slightly faster in the pre-group compared to the post (123 minutes compared to 191 minutes), this difference was not statistically different. Implementation resulted in no statistically significant impact on ED LOS, hospital LOS or escalation to a higher level of care due to bronchiolitis score implementation. The use of supplemental interventions like antibiotics, bronchodilators and steroids was not significantly reduced following the implementation of the scoring protocol. Minor differences in virus prevalence, viral testing patterns were seen. Patients with a bronchiolitis score ≥ 10 had a longer hospital length of stay, 58.9 hours (IQR 63.8) versus 40.2 hours (IQR 47.2), p=0.045. Conclusion(s): The implementation of a bronchiolitis scoring system within the pediatric ED did not impact HFNC use or correlate with increased ED LOS, hospital LOS, or escalation to higher levels of care.
Bingham CVBingham_CV 2021.pdf
The Utilization of High Flow Nasal Canula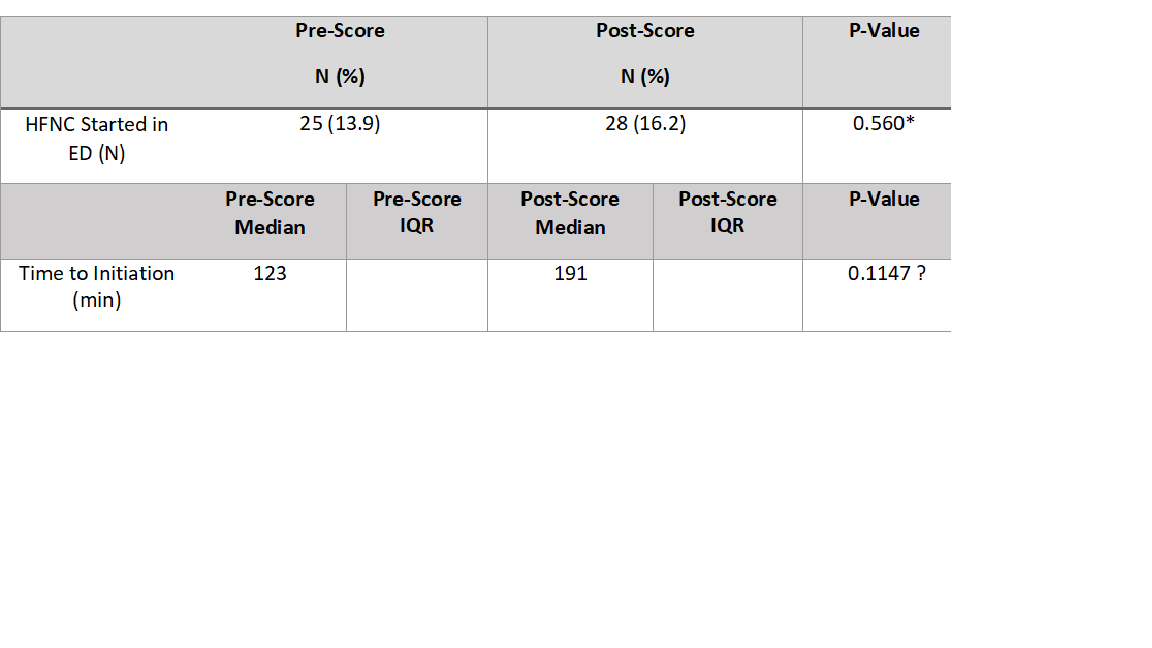 The comparison of HFNC utilization pre and post-scoring pathway implementation. * Chi Square test, significant difference defined as p < 0.05. ꝉ Wilcoxon Summed Rank Test, significant difference defined as p < 0.05.
The comparison of HFNC utilization pre and post-scoring pathway implementation. * Chi Square test, significant difference defined as p < 0.05. ꝉ Wilcoxon Summed Rank Test, significant difference defined as p < 0.05.
Objective: The aim of this study was to assess protocol implementation with throughput and quality metrics to include time to HFNC, ED length of stay (LOS), hospital LOS and rate of escalation to a higher level of service.
Design/Methods: We performed a retrospective chart review of patients under age 24 months admitted with bronchiolitis as their primary diagnosis. The data was collected November 2018-April 2019 (pre-implementation) and November 2019-April 2020 (post-implementation). The primary outcome measures included ED LOS, time to implementation of HFNC, hospital LOS and rate of escalation to a higher level of service. Secondary outcome measures included utilization of bronchodilators, antibiotics and steroids. Viral panel and chest radiograph acquisition and results were also correlated with bronchiolitis severity. Chi square test and Wilcoxon Rank Sum procedure with Kruskal-Wallis test were used to compare groups as appropriate.
Results: A total of 353 patients were included in this study, 180 in the pre-implementation group and 173 in the post-implementation group. HFNC was started in 25 patients in the pre-implementation group compared to 28 in the post-implementation (13.9% vs 16.2% respectively). The mean time to initiation of HFNC was slightly faster in the pre-group compared to the post (123 minutes compared to 191 minutes), this difference was not statistically different. Implementation resulted in no statistically significant impact on ED LOS, hospital LOS or escalation to a higher level of care due to bronchiolitis score implementation. The use of supplemental interventions like antibiotics, bronchodilators and steroids was not significantly reduced following the implementation of the scoring protocol. Minor differences in virus prevalence, viral testing patterns were seen. Patients with a bronchiolitis score ≥ 10 had a longer hospital length of stay, 58.9 hours (IQR 63.8) versus 40.2 hours (IQR 47.2), p=0.045. Conclusion(s): The implementation of a bronchiolitis scoring system within the pediatric ED did not impact HFNC use or correlate with increased ED LOS, hospital LOS, or escalation to higher levels of care.
Bingham CVBingham_CV 2021.pdf
The Utilization of High Flow Nasal Canula
 The comparison of HFNC utilization pre and post-scoring pathway implementation. * Chi Square test, significant difference defined as p < 0.05. ꝉ Wilcoxon Summed Rank Test, significant difference defined as p < 0.05.
The comparison of HFNC utilization pre and post-scoring pathway implementation. * Chi Square test, significant difference defined as p < 0.05. ꝉ Wilcoxon Summed Rank Test, significant difference defined as p < 0.05.