General Pediatrics: Primary Care/Prevention
Category: Abstract Submission
General Pediatrics I
594 - Inconsistency of Fluoride Varnish Application Guidelines for Children Under Five Years of Age in Primary Care Settings
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 594
Publication Number: 594.208
Publication Number: 594.208
Sarah L. Goff, University of Massachusetts, Amherst, AMHERST, MA, United States; Grace Gahlon, RAND Corporation, Baltimore, MD, United States; Kimberley Geissler, University of Massachusetts Amherst, Amherst, MA, United States; Andrew Dick, The RAND Corporation, Boston, MA, United States; Ashley M. Kranz, RAND Corporation, Arlington, VA, United States

Sarah Goff, PhD, MD
Associate Professor
University of Massachusetts Medical School
AMHERST, Massachusetts, United States
Presenting Author(s)
Background: Dental caries affects one-fourth of children aged 2-5 in the U.S, with Hispanic and Black children twice as likely to have caries. Since 2014, and reaffirmed in 2021, the United States Preventive Services Task Force (USPSTF) has recommended that primary care clinicians apply fluoride varnish (FV) to the teeth of all children under five years of age regardless of oral health risk or oral fluoride supplement status. However, fewer than 10% of eligible children receive FV as recommended. Inconsistent clinical practice guidelines (CPGs) and guidelines that have not been updated within five years are associated with lower delivery of evidence-based health interventions, and thus understanding consistency and timing of available CPGs is critical to assess barriers to FV applications in medical settings.
Objective: To determine variation in internet-accessible CPGs for FV application in primary care settings for children under five years of age.
Design/Methods: We identified CPGs published in the last 10 years that included recommendations for application of FV in primary care settings for children under the age of five years using the search terms fluoride varnish + [application; guidelines ¬or recommendations; children or pediatric; American Academy of Pediatrics; American Academy of Pediatric Dentistry; American Dental Association] and a search of Guideline Central. The date of the most recent version of the CPGs was documented and guidance regarding periodicity and eligibility in relation to oral health risk status, presence of a dental home, and receipt of oral fluoride supplementation were compared.
Results: Inclusive of the USPSTF recommendation, ten unique CPGs for FV application were identified from seven organizations. Two of the CPGs identified were published more than five years previously. Recommendations varied across organizations, as well as within organizations. Differences in CPGs’ recommendations were identified regarding periodicity as well as eligibility based on dental home and oral health risk factors (Table). The USPSTF recommends that FV be applied to the teeth of all young children, but six of the guidelines indicated that caries risk or presence of a dental home should be considered in determining whether or how often to apply FV.Conclusion(s): Inconsistent CPGs may contribute to the current low rates of delivering evidence-based FV application to children under five years of age in primary care settings. Centralized review and harmonization of CPGs for FV may help to address oral health disparities.
Clinical Practice Guidelines for Fluoride Varnish Application in Pediatric Primary Care (2011-21)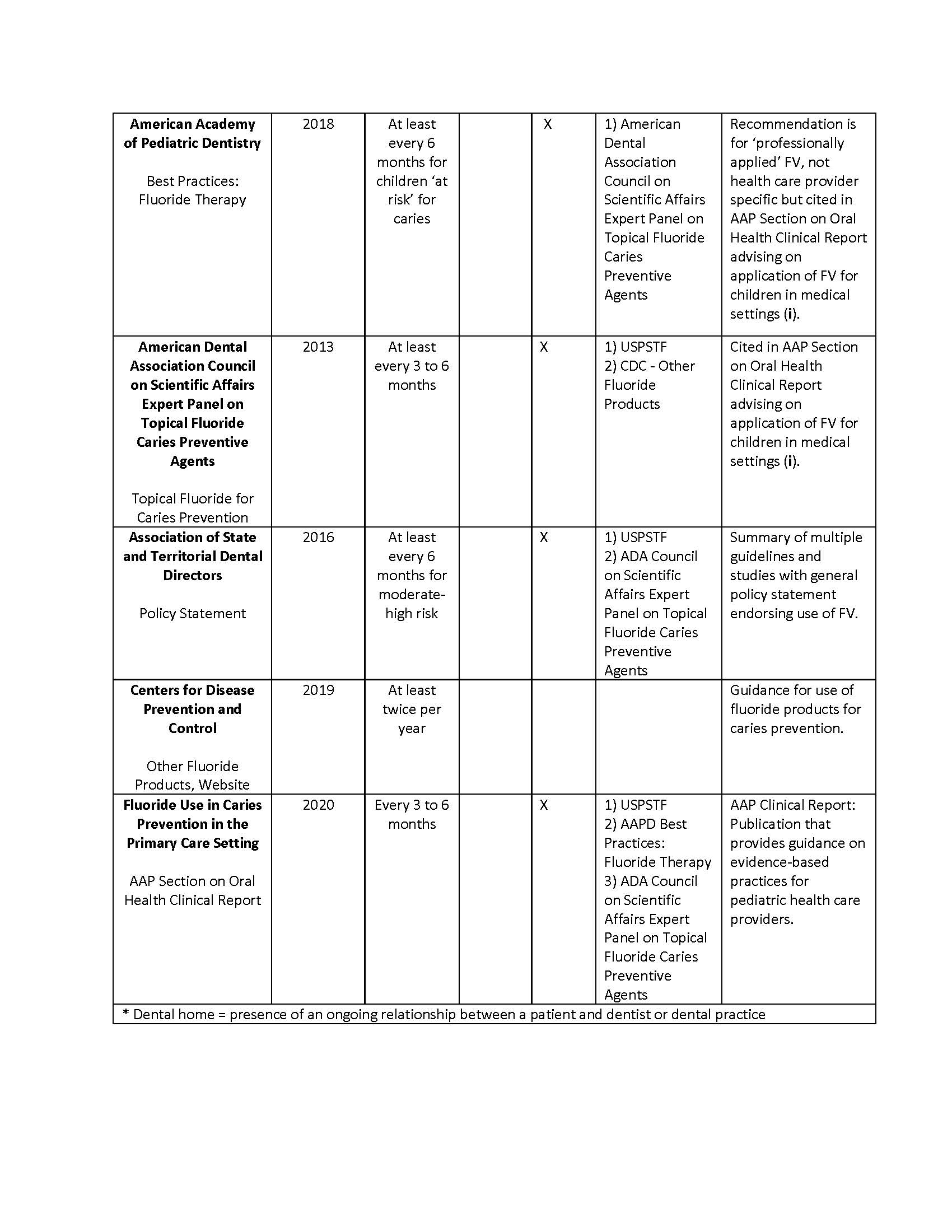
Objective: To determine variation in internet-accessible CPGs for FV application in primary care settings for children under five years of age.
Design/Methods: We identified CPGs published in the last 10 years that included recommendations for application of FV in primary care settings for children under the age of five years using the search terms fluoride varnish + [application; guidelines ¬or recommendations; children or pediatric; American Academy of Pediatrics; American Academy of Pediatric Dentistry; American Dental Association] and a search of Guideline Central. The date of the most recent version of the CPGs was documented and guidance regarding periodicity and eligibility in relation to oral health risk status, presence of a dental home, and receipt of oral fluoride supplementation were compared.
Results: Inclusive of the USPSTF recommendation, ten unique CPGs for FV application were identified from seven organizations. Two of the CPGs identified were published more than five years previously. Recommendations varied across organizations, as well as within organizations. Differences in CPGs’ recommendations were identified regarding periodicity as well as eligibility based on dental home and oral health risk factors (Table). The USPSTF recommends that FV be applied to the teeth of all young children, but six of the guidelines indicated that caries risk or presence of a dental home should be considered in determining whether or how often to apply FV.Conclusion(s): Inconsistent CPGs may contribute to the current low rates of delivering evidence-based FV application to children under five years of age in primary care settings. Centralized review and harmonization of CPGs for FV may help to address oral health disparities.
Clinical Practice Guidelines for Fluoride Varnish Application in Pediatric Primary Care (2011-21)

