Allergy, Immunology and Rheumatology
Category: Abstract Submission
Allergy, Immunology, and Rheumatology I
618 - Promoting Penicillin Allergy Documentation and Delabeling at a Pediatric Hospital
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 618
Publication Number: 618.200
Publication Number: 618.200
Alexandra Navard-Keck, Dell Medical School at The University of Texas Austin, Austin, TX, United States; Julia Sapozhnikov, Loyola University Chicago Stritch School of Medicine, Chicago, IL, United States; Kyle Robillard, University of Texas at Austin Dell Medical School, Austin, TX, United States; Pooja Varshney, University of Texas at Austin Dell Medical School, Austin, TX, United States; Marisol Fernandez, Dell Children's Medical Center of Central Texas, Austin, TX, United States

Alexandra Navard-Keck, MD
Pediatric Resident
Dell Medical School at The University of Texas Austin
Austin, Texas, United States
Presenting Author(s)
Background: Approximately 15-20% of the US population carries a penicillin allergy label; however, 95% do not have a current penicillin allergy. Penicillin allergy labels are associated with many personal and public health implications. Individually, penicillin allergy labels are associated with fewer antibiotic choices, less effective alternatives, the use of broader-spectrum antibiotics, and more toxic side effects. Public health implications include increased antimicrobial resistance, higher rates of C. difficile, increased healthcare costs, and longer hospital lengths of stay.
Objective: This project aims to improve penicillin allergy documentation and delabel patients at minimal risk for a reaction at a children’s hospital.
Design/Methods: This was a single-center, uncontrolled pre-post quasi-experimental study at an urban pediatric teaching hospital. Interventions included clinician education, development of clinical tools, and ongoing feedback. Input from key stakeholders in infectious disease, allergy, pharmacy, and nursing was utilized. Patients with a penicillin allergy were included. Patients admitted to the intensive care unit were excluded. Primary outcomes were the presence and quality of penicillin allergy documentation in the electronic healthcare record. Documentation quality was assessed on a scale of 0-3 based on inclusion of reaction onset, description, and timing. Secondary outcomes were percent of patients receiving the first-line antibiotic for community acquired pneumonia, acute otitis media, skin and soft tissue infections, and urinary tract infections. Statistical analysis was done with chi-square testing.
Results: In the pre-intervention group (n=372), a reaction to penicillin was documented in 29% of patients, and 1% had high quality documentation. Common documented reactions were rash (62%) and hives (26%). No patients were delabled. Patients in the post-intervention group (n=80) were more likely to have a reaction to penicillin documented (55%, p=0.003) and have higher quality documentation (80%, p< 0.00001). The variety of documented reaction types increased: rash (35%), hives (23.5%), anaphylaxis (5%), mucocutaneous reaction (5%), DRESS (5%), isolated GI symptoms (5%) and other (20%). Twenty-six percent of patients were appropriately delabeled. Amoxicillin use increased and third generation cephalosporin use decreased for the observed disease states.Conclusion(s): Clinician education and clinical tools focused on improving appropriate history-taking and documentation improve penicillin allergy management. Patients with minimal risk of a penicillin allergy should be delabeled.
Penicillin Allergy Documentation Pre- and Post-Intervention.png) Post-intervention, there was increased detail of documentation, including severe reactions, and improved appropriate delabeling rates.
Post-intervention, there was increased detail of documentation, including severe reactions, and improved appropriate delabeling rates.
Documentation Quality Over Time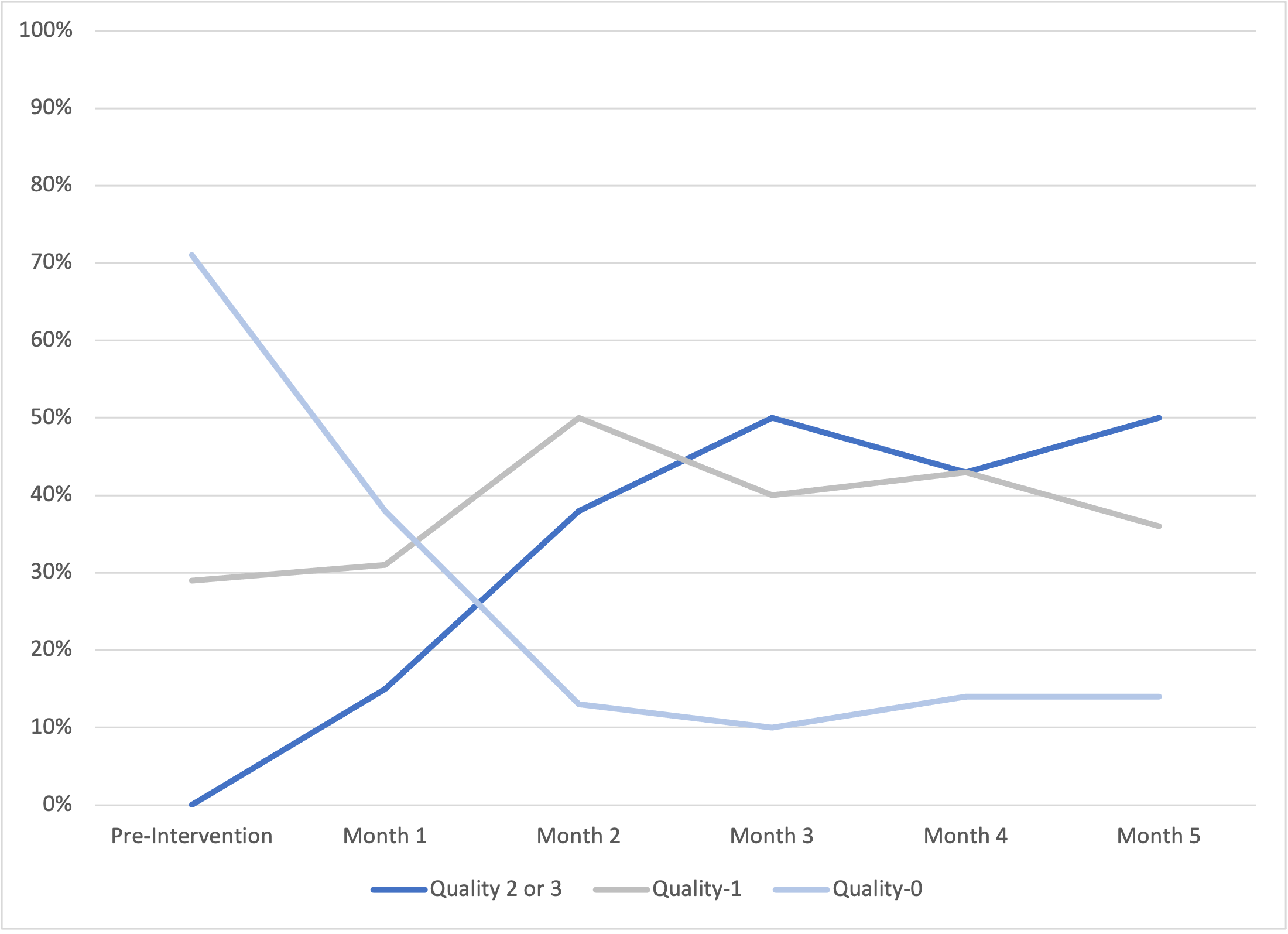 Documentation quality improved post-intervention. Quality was rated on a scale of 0-3 based on the inclusion of reaction description, onset, and timing. One point was added for each component present. High quality documentation was defined as Quality 2 or 3.
Documentation quality improved post-intervention. Quality was rated on a scale of 0-3 based on the inclusion of reaction description, onset, and timing. One point was added for each component present. High quality documentation was defined as Quality 2 or 3.
Objective: This project aims to improve penicillin allergy documentation and delabel patients at minimal risk for a reaction at a children’s hospital.
Design/Methods: This was a single-center, uncontrolled pre-post quasi-experimental study at an urban pediatric teaching hospital. Interventions included clinician education, development of clinical tools, and ongoing feedback. Input from key stakeholders in infectious disease, allergy, pharmacy, and nursing was utilized. Patients with a penicillin allergy were included. Patients admitted to the intensive care unit were excluded. Primary outcomes were the presence and quality of penicillin allergy documentation in the electronic healthcare record. Documentation quality was assessed on a scale of 0-3 based on inclusion of reaction onset, description, and timing. Secondary outcomes were percent of patients receiving the first-line antibiotic for community acquired pneumonia, acute otitis media, skin and soft tissue infections, and urinary tract infections. Statistical analysis was done with chi-square testing.
Results: In the pre-intervention group (n=372), a reaction to penicillin was documented in 29% of patients, and 1% had high quality documentation. Common documented reactions were rash (62%) and hives (26%). No patients were delabled. Patients in the post-intervention group (n=80) were more likely to have a reaction to penicillin documented (55%, p=0.003) and have higher quality documentation (80%, p< 0.00001). The variety of documented reaction types increased: rash (35%), hives (23.5%), anaphylaxis (5%), mucocutaneous reaction (5%), DRESS (5%), isolated GI symptoms (5%) and other (20%). Twenty-six percent of patients were appropriately delabeled. Amoxicillin use increased and third generation cephalosporin use decreased for the observed disease states.Conclusion(s): Clinician education and clinical tools focused on improving appropriate history-taking and documentation improve penicillin allergy management. Patients with minimal risk of a penicillin allergy should be delabeled.
Penicillin Allergy Documentation Pre- and Post-Intervention
.png) Post-intervention, there was increased detail of documentation, including severe reactions, and improved appropriate delabeling rates.
Post-intervention, there was increased detail of documentation, including severe reactions, and improved appropriate delabeling rates.Documentation Quality Over Time
 Documentation quality improved post-intervention. Quality was rated on a scale of 0-3 based on the inclusion of reaction description, onset, and timing. One point was added for each component present. High quality documentation was defined as Quality 2 or 3.
Documentation quality improved post-intervention. Quality was rated on a scale of 0-3 based on the inclusion of reaction description, onset, and timing. One point was added for each component present. High quality documentation was defined as Quality 2 or 3.