Neonatal Neurology: Basic
Category: Abstract Submission
Neurology 2: Basic-Translational
405 - Surfactant Protein A and Neuroinflammation Using Three Models in Neonatal Mice
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 405
Publication Number: 405.236
Publication Number: 405.236
Caroline E. Crocker, University of Texas Health Science Center at Houston, Houston, TX, United States; Romana sharmeen, University of Texas Health Science Center at Houston, pearland, TX, United States; Joseph Alcorn, UT Medical School at Houston, Houston, TX, United States

Caroline E. Crocker, DO
Neonatology Fellow
University of Texas Health Science Center at Houston
Houston, Texas, United States
Presenting Author(s)
Background: Surfactant protein A (SP-A) has a well-established role in pulmonary innate immunity and attenuation of inflammation. SP-A has also been shown to modulate inflammation at extrapulmonary sites, including the brain, using various models of inflammatory disease. Expression of SP-A has previously been reported in rat and human brain but has not been investigated in mouse brain.
Objective: To determine if SP-A acts as an immunomodulatory protein to attenuate inflammation in the neonatal mouse brain.
Design/Methods: mRNA isolated from brain and lung tissue from wildtype (WT) and SP-A deficient (SP-A-/-) C57BL/6 mice at 2 and 6 wks of age was subjected to RT-PCR analysis (50 cycles) to detect SP-A expression. Three models of neonatal brain inflammation were used in postnatal day 7 (P7) WT and SP-A-/- neonatal mice: sepsis, intraventricular hemorrhage (IVH), and hypoxic-ischemic encephalopathy (HIE). In the sepsis model, lipopolysaccharide (LPS) or saline was injected intraperitoneally. In the IVH model, pups underwent unilateral intraventricular injection with hemoglobin or saline. In the HIE model, pups underwent unilateral common carotid artery ligation or sham surgery followed by exposure to systemic hypoxia. Pups were euthanized 24 hrs following injection or surgery, and mRNA was isolated from brain tissue. Cytokine expression (CXCL1, IL-1β, IL-6, TNF-a, and IL-10) was then determined by real-time quantitative RT-PCR analysis.
Results: While expression of SP-A mRNA was detected in lung tissue of WT mice, it was not detected in the lungs of SP-A-/- mice nor in the brains of either WT or SP-A-/- mice. At 24 hours post-LPS injection, expression of all cytokine mRNAs was significantly increased in the brains of SP-A-/- mice compared with WT mice. Similarly, expression of all cytokine mRNAs was significantly increased in the brains of SP-A-/- mice compared with WT mice at 4 hrs following intraventricular hemoglobin injection, with the exception of IL-6. Finally, expression of all pro-inflammatory cytokine mRNAs was significantly increased in the brains of SP-A-/- mice compared with WT mice at 24 hrs following HIE surgery with hypoxia exposure.Conclusion(s): We could not detect SP-A expression in murine brain tissue. Despite this finding, neonatal mice deficient in SP-A showed significantly greater cytokine expression in the brain compared to neonatal WT mice subjected to three different models of neuroinflammation. These results suggest that SP-A-/- mice are more susceptible to neuroinflammation than WT mice, thus supporting the original hypothesis that SP-A attenuates inflammation in the neonatal mouse brain.
Caroline Crocker Curriculum VitaeCrocker-CV Updated 2021-12-21.pdf
Figure 2. Real-time quantitative RT-PCR analysis of cytokine mRNA following IVH model.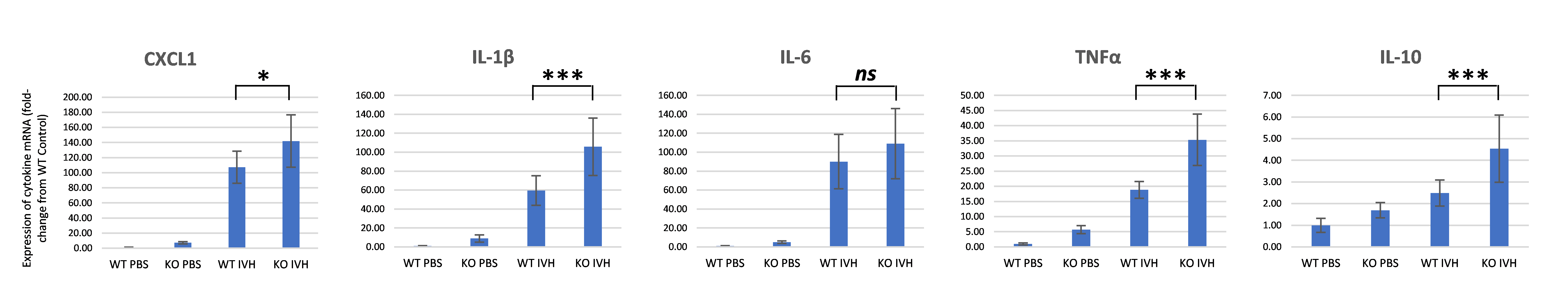 Expression of all cytokine mRNAs was significantly increased in the brains of SP-A-/- (KO) mice compared with WT mice at 4 hours following intraventricular hemoglobin injection, with the exception of IL-6.
Expression of all cytokine mRNAs was significantly increased in the brains of SP-A-/- (KO) mice compared with WT mice at 4 hours following intraventricular hemoglobin injection, with the exception of IL-6.
Objective: To determine if SP-A acts as an immunomodulatory protein to attenuate inflammation in the neonatal mouse brain.
Design/Methods: mRNA isolated from brain and lung tissue from wildtype (WT) and SP-A deficient (SP-A-/-) C57BL/6 mice at 2 and 6 wks of age was subjected to RT-PCR analysis (50 cycles) to detect SP-A expression. Three models of neonatal brain inflammation were used in postnatal day 7 (P7) WT and SP-A-/- neonatal mice: sepsis, intraventricular hemorrhage (IVH), and hypoxic-ischemic encephalopathy (HIE). In the sepsis model, lipopolysaccharide (LPS) or saline was injected intraperitoneally. In the IVH model, pups underwent unilateral intraventricular injection with hemoglobin or saline. In the HIE model, pups underwent unilateral common carotid artery ligation or sham surgery followed by exposure to systemic hypoxia. Pups were euthanized 24 hrs following injection or surgery, and mRNA was isolated from brain tissue. Cytokine expression (CXCL1, IL-1β, IL-6, TNF-a, and IL-10) was then determined by real-time quantitative RT-PCR analysis.
Results: While expression of SP-A mRNA was detected in lung tissue of WT mice, it was not detected in the lungs of SP-A-/- mice nor in the brains of either WT or SP-A-/- mice. At 24 hours post-LPS injection, expression of all cytokine mRNAs was significantly increased in the brains of SP-A-/- mice compared with WT mice. Similarly, expression of all cytokine mRNAs was significantly increased in the brains of SP-A-/- mice compared with WT mice at 4 hrs following intraventricular hemoglobin injection, with the exception of IL-6. Finally, expression of all pro-inflammatory cytokine mRNAs was significantly increased in the brains of SP-A-/- mice compared with WT mice at 24 hrs following HIE surgery with hypoxia exposure.Conclusion(s): We could not detect SP-A expression in murine brain tissue. Despite this finding, neonatal mice deficient in SP-A showed significantly greater cytokine expression in the brain compared to neonatal WT mice subjected to three different models of neuroinflammation. These results suggest that SP-A-/- mice are more susceptible to neuroinflammation than WT mice, thus supporting the original hypothesis that SP-A attenuates inflammation in the neonatal mouse brain.
Caroline Crocker Curriculum VitaeCrocker-CV Updated 2021-12-21.pdf
Figure 2. Real-time quantitative RT-PCR analysis of cytokine mRNA following IVH model.
 Expression of all cytokine mRNAs was significantly increased in the brains of SP-A-/- (KO) mice compared with WT mice at 4 hours following intraventricular hemoglobin injection, with the exception of IL-6.
Expression of all cytokine mRNAs was significantly increased in the brains of SP-A-/- (KO) mice compared with WT mice at 4 hours following intraventricular hemoglobin injection, with the exception of IL-6.