Neonatal Fetal Nutrition & Metabolism
Category: Abstract Submission
Neonatal Fetal Nutrition & Metabolism IV
454 - The Association Between BMI Trajectories and Bronchopulmonary Dysplasia Among Preterm Infants Born Less Than 30 Weeks Gestational Age
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 454
Publication Number: 454.335
Publication Number: 454.335
Laura Li Ching Ng, Montreal Children Hospital, Montréal, PQ, Canada; Sharina Patel, McGill University Health Centre Research Institute, Montreal, PQ, Canada; Marie-Eve Besner, Montreal Children's Hospital, Montreal, PQ, Canada; Hugues Plourde, McGill University, Montreal, PQ, Canada; Anie Lapointe, CHU Ste-Justine, Montréal, PQ, Canada; Victoria Bizgu, McGill University Faculty of Medicine and Health Sciences, Cote-Saint-Luc, PQ, Canada; Guilherme Sant'Anna, McGill University Health Center, MONTREAL, PQ, Canada; Marc Beltempo, McGill University, Montreal, PQ, Canada
- LL
Laura Li Ching Ng, RD, CNSC
Registered Dietitian
Montreal Children Hospital
Montréal, Quebec, Canada
Presenting Author(s)
Background: Most nutritional interventions aim to optimize weight gain among preterm infants. However, disproportionate growth resulting from changes in body mass index (BMI) may increase the need for respiratory support and subsequently the risk of bronchopulmonary dysplasia (BPD).
Objective: To determine the association between BMI growth trajectory from birth to 36 weeks corrected gestational age (CGA) and BPD among infants born < 30 weeks gestational age (GA).
Design/Methods: Multicenter retrospective cohort study including infants born < 30 weeks GA admitted to 3 tertiary neonatal intensive care units from 2015-2018 that survived ≥14 days. Infant characteristics and outcomes were collected from the Canadian Neonatal Network database and biweekly anthropometric measurements and caloric intake from medical chart review. The primary outcome was BPD (need for respiratory support or oxygen at 36 weeks CGA). Change in BMI z scores (ΔBMI) were calculated from birth to 36 weeks CGA using the 2015 BMI Olsen curves and grouped into quartiles of change. Weight and length z scores were calculated using 2013 Fenton curves. The association of ΔBMI quartile with BPD was assessed using generalized linear mixed models adjusted for confounders.
Results: Among 772 infants, median GA was 27 weeks [IQR 26;29] and 391 (51%) developed BPD. BPD and BPD-free infants had similar birth BMI z scores (median 0.19 [IQR -0.66; 0.95] vs 0.31 [-0.34; 0.91], P=0.10). At 36 weeks CGA, BPD infants had higher increase in ΔBMI z score when compared to BPD-free infants (median 0.16 [IQR -0.64; 1.03] vs -0.29 [-1.03; 0.49], P < 0.01). From birth to 36 weeks CGA, BPD infants experienced less decrease in weight z score (Δweight z score, median -0.76 [IQR -1.29; -0.27] vs -0.90 [-1.30; -0.50], P < 0.01) compared to BPD-free infants despite a slower linear growth (Δlength z score, -1.09 [-1.78; -0.44] vs -0.97 [-1.52; -0.37], P=0.03) and similar mean caloric intakes (124 kcal/kg/d vs 123 kcal/kg/d, P=0.14). In the adjusted analyses, higher quartiles of ΔBMI were associated with higher odds of BPD compared to the 2nd quartile (Q3 vs Q2, OR 2.03, 95%CI 1.26-3.28) and (Q4 vs Q2, OR 1.85, 95%CI 1.12-3.03).Conclusion(s): An increase in ΔBMI z score from birth to 36 weeks CGA was associated with higher odds of BPD among infants born < 30 weeks GA. This increase in BMI may reflect disproportionate growth (higher weight gain but lack of linear growth) despite similar caloric intake, suggesting that some infants with evolving BPD may require individualized nutritional requirements and growth targets.
Table 1. Maternal and neonatal characteristics of full cohort, BPD and BPD-free infants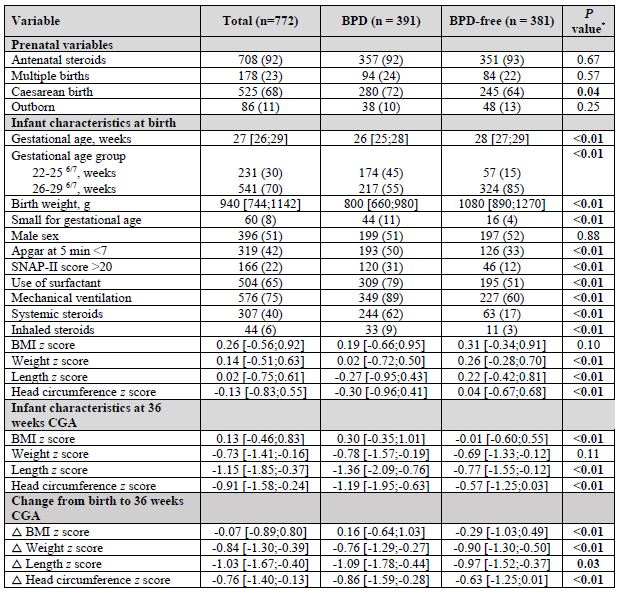 Legend: Data presented as n (%) or median [IQR, interquartile range]
Legend: Data presented as n (%) or median [IQR, interquartile range]
*P values for comparisons between BPD and BPD-free infants were derived from the Chi-square test for categorical variables and Wilcoxon Rank Sum test for continuous variables
Table 2. Association of BPD with quartiles of BMI z score change from birth to 36 weeks CGA
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/16020/FGOVBGGC-1174300-2-IMG.jpg width=440 hheight=82.2295081967213 border=0 style=border-style: none;>Legend: Site was included as random effect in all models. Postnatal model adjustment variables: antenatal steroids, GA at birth, sex, multiple pregnancy, cesarian delivery, SGA status, SNAP-II score >20 and mechanical ventilation during admission
Objective: To determine the association between BMI growth trajectory from birth to 36 weeks corrected gestational age (CGA) and BPD among infants born < 30 weeks gestational age (GA).
Design/Methods: Multicenter retrospective cohort study including infants born < 30 weeks GA admitted to 3 tertiary neonatal intensive care units from 2015-2018 that survived ≥14 days. Infant characteristics and outcomes were collected from the Canadian Neonatal Network database and biweekly anthropometric measurements and caloric intake from medical chart review. The primary outcome was BPD (need for respiratory support or oxygen at 36 weeks CGA). Change in BMI z scores (ΔBMI) were calculated from birth to 36 weeks CGA using the 2015 BMI Olsen curves and grouped into quartiles of change. Weight and length z scores were calculated using 2013 Fenton curves. The association of ΔBMI quartile with BPD was assessed using generalized linear mixed models adjusted for confounders.
Results: Among 772 infants, median GA was 27 weeks [IQR 26;29] and 391 (51%) developed BPD. BPD and BPD-free infants had similar birth BMI z scores (median 0.19 [IQR -0.66; 0.95] vs 0.31 [-0.34; 0.91], P=0.10). At 36 weeks CGA, BPD infants had higher increase in ΔBMI z score when compared to BPD-free infants (median 0.16 [IQR -0.64; 1.03] vs -0.29 [-1.03; 0.49], P < 0.01). From birth to 36 weeks CGA, BPD infants experienced less decrease in weight z score (Δweight z score, median -0.76 [IQR -1.29; -0.27] vs -0.90 [-1.30; -0.50], P < 0.01) compared to BPD-free infants despite a slower linear growth (Δlength z score, -1.09 [-1.78; -0.44] vs -0.97 [-1.52; -0.37], P=0.03) and similar mean caloric intakes (124 kcal/kg/d vs 123 kcal/kg/d, P=0.14). In the adjusted analyses, higher quartiles of ΔBMI were associated with higher odds of BPD compared to the 2nd quartile (Q3 vs Q2, OR 2.03, 95%CI 1.26-3.28) and (Q4 vs Q2, OR 1.85, 95%CI 1.12-3.03).Conclusion(s): An increase in ΔBMI z score from birth to 36 weeks CGA was associated with higher odds of BPD among infants born < 30 weeks GA. This increase in BMI may reflect disproportionate growth (higher weight gain but lack of linear growth) despite similar caloric intake, suggesting that some infants with evolving BPD may require individualized nutritional requirements and growth targets.
Table 1. Maternal and neonatal characteristics of full cohort, BPD and BPD-free infants
 Legend: Data presented as n (%) or median [IQR, interquartile range]
Legend: Data presented as n (%) or median [IQR, interquartile range]*P values for comparisons between BPD and BPD-free infants were derived from the Chi-square test for categorical variables and Wilcoxon Rank Sum test for continuous variables
Table 2. Association of BPD with quartiles of BMI z score change from birth to 36 weeks CGA
<img src=https://www.abstractscorecard.com/uploads/Tasks/upload/16020/FGOVBGGC-1174300-2-IMG.jpg width=440 hheight=82.2295081967213 border=0 style=border-style: none;>Legend: Site was included as random effect in all models. Postnatal model adjustment variables: antenatal steroids, GA at birth, sex, multiple pregnancy, cesarian delivery, SGA status, SNAP-II score >20 and mechanical ventilation during admission
