Clinical Bioethics
Category: Abstract Submission
Clinical Bioethics
616 - Trial of Therapy on Trial: Inconsistent Thresholds for Withdrawal of Life-sustaining Therapies in the NICU
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 616
Publication Number: 616.306
Publication Number: 616.306
Jacqueline Meadow, Lurie Children's Hospital, Chicago, IL, United States; Natalia Henner, Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL, United States; Karen Rychlik, Northwestern University, Chicago, IL, United States

Jacqueline Meadow, MD
Neonatology Fellow
Lurie Children's Hospital
Chicago, Illinois, United States
Presenting Author(s)
Background: Perinatal counseling at the borders of gestational viability includes discussions of predicted morbidity and mortality, which may frame initial resuscitation decisions. This informed decision-making is critical to informed consent. In peri-viability counseling, three care options are often presented to parents: non-intervention, full intervention, or “a trial of therapy.” The latter is framed as an attempt at initial resuscitation, followed by ongoing assessment of infant’s clinical course, and a possibility of withdrawing life-sustaining therapies (LST) if the projected outcome due to accumulated morbidities becomes unacceptable to parents. Little is known about clinical criteria or neonatal morbidities neonatologists use to signal the possible end of a trial of therapy. Inconsistency in counseling and practices threatens the validity of the informed consent process surrounding the decision to resuscitate a peri-viable infant.
Objective: Describe neonatologists’ practice in discussing withdrawal of LST at the borders of gestational viability.
Design/Methods: A survey describing five clinical scenarios, which varied across two gestational age groups and distinct common morbidities, was sent to attending neonatologists at a large, urban, level IV NICU. Participants rated their likelihood to discuss an option to withdraw LST on a scale of 1 (not at all likely) to 10 (extremely likely).
Results: With an 89% response rate (32/36), half (5/10) of all questions had ranges from 1 (not at all likely) to 10 (extremely likely), demonstrating extremely wide variability in counseling practices. The scenario in which neonatologists were most likely to discuss an option to withdraw LST was a 22-23 week infant with bilateral severe intra-ventricular hemorrhage (24/32). The presence of additional co-morbidities increased the likelihood of discussing withdrawal of LST. Lower gestational age was associated with higher likelihood to discuss withdrawal of LST in all scenarios.Conclusion(s): Neonatologists have a highly variable practice pertaining to end-of-life care decisions when presented with theoretical scenarios. The second phase of our study will demonstrate a similar practice variation in clinical encounters. Parents may be unaware of this variability when electing for a trial of therapy on behalf of their infants, questioning the validity of informed consent surrounding resuscitation of peri-viable infants.
Table 1 - Descriptive Statistics of Responses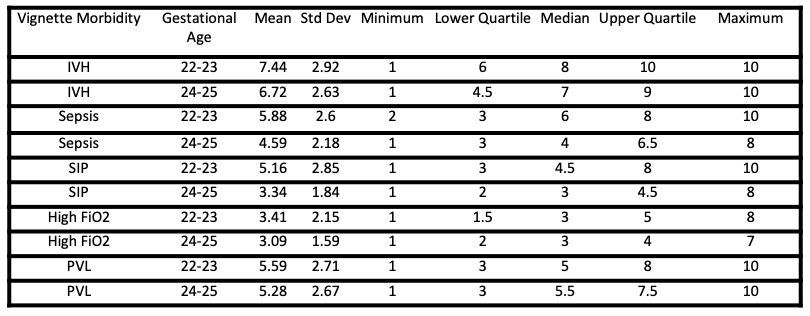 Responses to question “How likely would you be to discuss limiting or ending a “trial of therapy” for the following clinical scenarios? 1: Not at all likely – 10: Extremely likely”
Responses to question “How likely would you be to discuss limiting or ending a “trial of therapy” for the following clinical scenarios? 1: Not at all likely – 10: Extremely likely”
Figure 1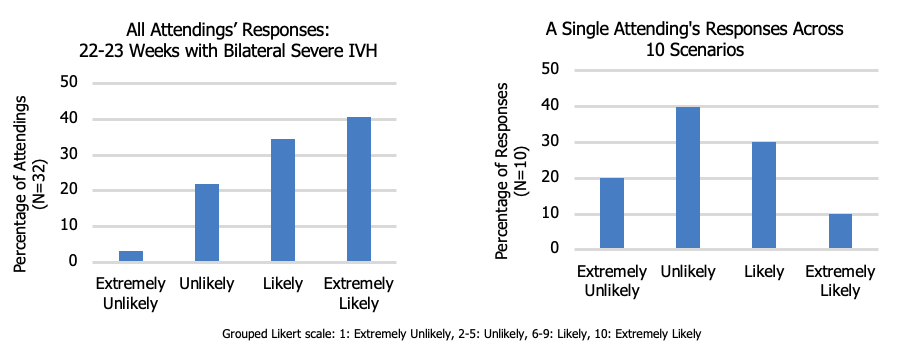 Variability in response of likelihood to discuss withdrawal of life-sustaining therapies
Variability in response of likelihood to discuss withdrawal of life-sustaining therapies
Objective: Describe neonatologists’ practice in discussing withdrawal of LST at the borders of gestational viability.
Design/Methods: A survey describing five clinical scenarios, which varied across two gestational age groups and distinct common morbidities, was sent to attending neonatologists at a large, urban, level IV NICU. Participants rated their likelihood to discuss an option to withdraw LST on a scale of 1 (not at all likely) to 10 (extremely likely).
Results: With an 89% response rate (32/36), half (5/10) of all questions had ranges from 1 (not at all likely) to 10 (extremely likely), demonstrating extremely wide variability in counseling practices. The scenario in which neonatologists were most likely to discuss an option to withdraw LST was a 22-23 week infant with bilateral severe intra-ventricular hemorrhage (24/32). The presence of additional co-morbidities increased the likelihood of discussing withdrawal of LST. Lower gestational age was associated with higher likelihood to discuss withdrawal of LST in all scenarios.Conclusion(s): Neonatologists have a highly variable practice pertaining to end-of-life care decisions when presented with theoretical scenarios. The second phase of our study will demonstrate a similar practice variation in clinical encounters. Parents may be unaware of this variability when electing for a trial of therapy on behalf of their infants, questioning the validity of informed consent surrounding resuscitation of peri-viable infants.
Table 1 - Descriptive Statistics of Responses
 Responses to question “How likely would you be to discuss limiting or ending a “trial of therapy” for the following clinical scenarios? 1: Not at all likely – 10: Extremely likely”
Responses to question “How likely would you be to discuss limiting or ending a “trial of therapy” for the following clinical scenarios? 1: Not at all likely – 10: Extremely likely”Figure 1
 Variability in response of likelihood to discuss withdrawal of life-sustaining therapies
Variability in response of likelihood to discuss withdrawal of life-sustaining therapies