Neonatal Pulmonology
Category: Abstract Submission
Neonatal Pulmonology I: Antenatal Exposures
423 - Effects of Antenatal Steroids on Respiratory Outcomes in Infants 30-36 Weeks Gestation
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 423
Publication Number: 423.429
Publication Number: 423.429
Michael Kuzniewicz, Kaiser Permanante, Los Gatos, CA, United States; Sherian Li, Kaiser Permanente, San Ramon, CA, United States; Eileen M. Walsh, Kaiser Permanente Northern California, Oakland, CA, United States; Olivier Danhaive, Catholic University of Louvain, Brussels, Brabant Wallon, Belgium

Michael Kuzniewicz, MD MPH
Neonatologist/Research Scientist II
Kaiser Permanante Northern California
Los Gatos, California, United States
Presenting Author(s)
Background: Quantifying respiratory outcomes in infants 30-36 weeks gestation is important as clinicians compare the short-term benefits of antenatal steroids (ANS) with potential risks of neurodevelopmental disorders in this older gestational age group.
Objective: In a large population-based cohort, our objective was to characterize respiratory support in infants born at 30-36 weeks gestation and examine the impact of ANS on short-term outcomes.
Design/Methods:
We performed a cohort study of infants born at 14 Kaiser Permanente Northern California facilities between 2012-2018 born at 30-36 weeks. To determine the mode and duration of respiratory support, we used respiratory therapy flowsheets. Short-term outcomes included type and duration of respiratory support, surfactant administration, death, chronic lung disease, air leak syndromes, and pulmonary hemorrhage. We used logistic regression to evaluate the association between ANS and the outcomes, adjusting for gestational age, sex, cesarean delivery, race/ethnicity, maternal diabetes, pre-eclampsia, and small for gestational age.
Results:
In 19,810 infants born at 30-36 weeks gestation, 23.7% received some form of respiratory support. The predominant maximal form of support was NCPAP in 71.0% of the infants. Only 2.0% received invasive ventilation and 2.1% received surfactant, mostly in the 30-32 weeks group. Of those receiving respiratory support, the median duration of support was 70.2 hours (Interquartile range 29.2 to 146.1 hours). ANS prior to delivery were protective for any respiratory support for ≥ 3 days, NCPAP ≥ 3 days, surfactant, and mechanical ventilation. When restricted to infants 34-36 weeks, ANS prior to delivery were protective only for any respiratory support for ≥ 3 days. When examining the timing of ANS in this group, ANS given 48-168 hour prior to delivery was protective, but not those given < 48 hours or >168 hours. Overall, 32.7% received ANS, but only 6.6% in the window 48-168 hours prior to delivery.
Conclusion(s):
Conclusions: Respiratory support after birth was common in infants born at 30-36 weeks gestation but usually limited to non-invasive support, lasting just a few days. Overall, ANS reduced the risk of respiratory support and invasive treatments, but in infants 34-36 weeks had more limited effects and were only protective if administered 48-168 hour prior to delivery. The benefits of ANS in this group should be carefully weighed against potential adverse developmental effects.
TABLE 1: Highest Level of Respiratory Support in the 1st 3 days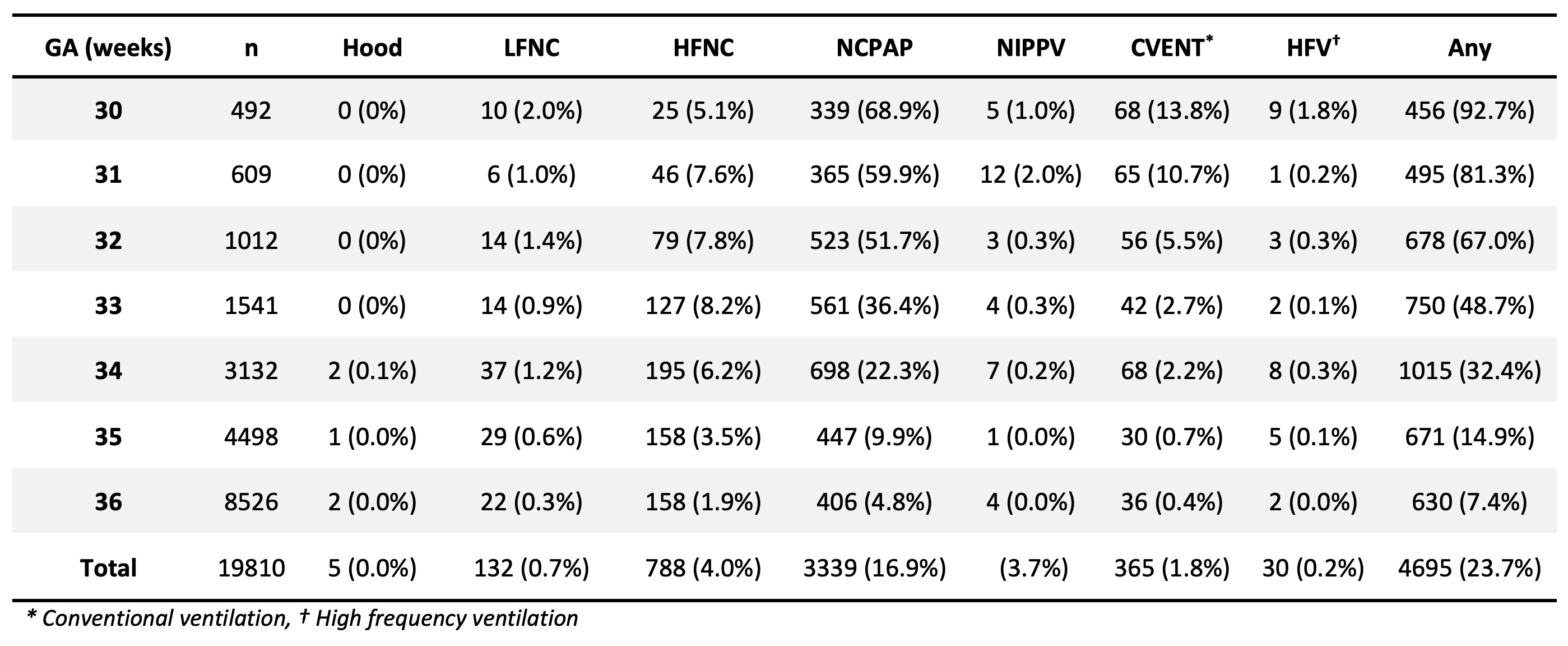
Table 2: Short Term Outcomes of Antenatal Steroids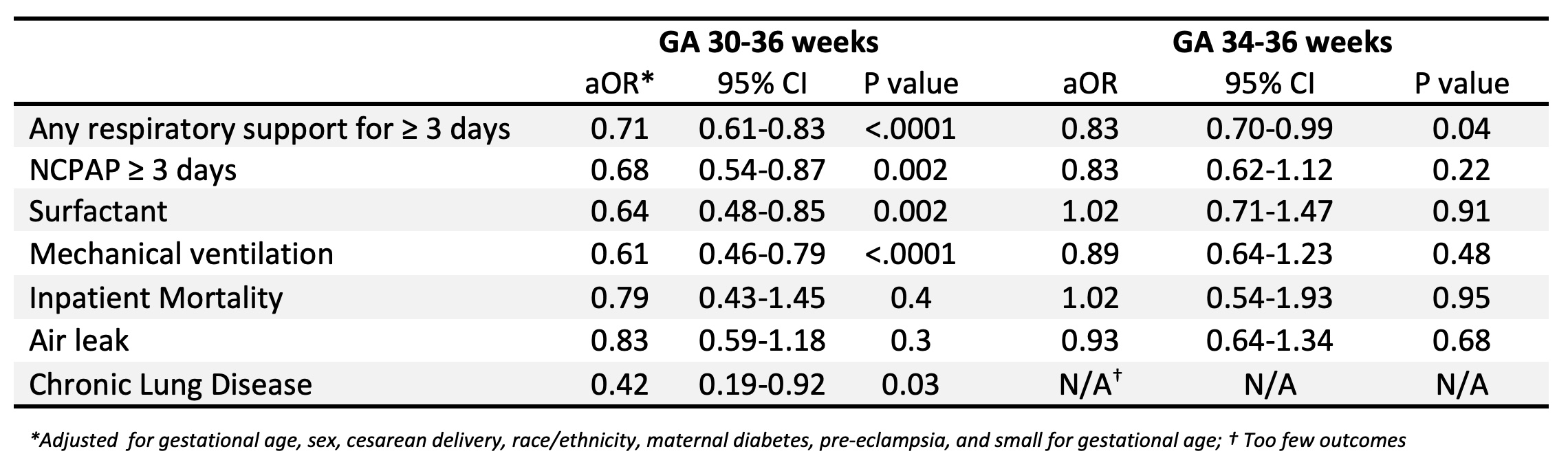
Objective: In a large population-based cohort, our objective was to characterize respiratory support in infants born at 30-36 weeks gestation and examine the impact of ANS on short-term outcomes.
Design/Methods:
We performed a cohort study of infants born at 14 Kaiser Permanente Northern California facilities between 2012-2018 born at 30-36 weeks. To determine the mode and duration of respiratory support, we used respiratory therapy flowsheets. Short-term outcomes included type and duration of respiratory support, surfactant administration, death, chronic lung disease, air leak syndromes, and pulmonary hemorrhage. We used logistic regression to evaluate the association between ANS and the outcomes, adjusting for gestational age, sex, cesarean delivery, race/ethnicity, maternal diabetes, pre-eclampsia, and small for gestational age.
Results:
In 19,810 infants born at 30-36 weeks gestation, 23.7% received some form of respiratory support. The predominant maximal form of support was NCPAP in 71.0% of the infants. Only 2.0% received invasive ventilation and 2.1% received surfactant, mostly in the 30-32 weeks group. Of those receiving respiratory support, the median duration of support was 70.2 hours (Interquartile range 29.2 to 146.1 hours). ANS prior to delivery were protective for any respiratory support for ≥ 3 days, NCPAP ≥ 3 days, surfactant, and mechanical ventilation. When restricted to infants 34-36 weeks, ANS prior to delivery were protective only for any respiratory support for ≥ 3 days. When examining the timing of ANS in this group, ANS given 48-168 hour prior to delivery was protective, but not those given < 48 hours or >168 hours. Overall, 32.7% received ANS, but only 6.6% in the window 48-168 hours prior to delivery.
Conclusion(s):
Conclusions: Respiratory support after birth was common in infants born at 30-36 weeks gestation but usually limited to non-invasive support, lasting just a few days. Overall, ANS reduced the risk of respiratory support and invasive treatments, but in infants 34-36 weeks had more limited effects and were only protective if administered 48-168 hour prior to delivery. The benefits of ANS in this group should be carefully weighed against potential adverse developmental effects.
TABLE 1: Highest Level of Respiratory Support in the 1st 3 days

Table 2: Short Term Outcomes of Antenatal Steroids

