Neonatal GI Physiology & NEC
Category: Abstract Submission
Neonatal GI Physiology & NEC III
505 - Effects of Human-Milk-Based Human-Milk Fortifier on Necrotizing Enterocolitis and Nutritional Outcomes in Preterm Infants
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 505
Publication Number: 505.426
Publication Number: 505.426
Shruti Patel, UMMS, Elkridge, MD, United States; Nikitha Mangalapally, University of Maryland, Baltimore, MD, United States; Alexandra Vlk, University of Maryland MSTP, Cockeysville, MD, United States; Christine Capriolo, University of Maryland School of Medicine, Baltimore, MD, United States; Erin E. Schofield, University of Maryland School of Medicine, Crownsville, MD, United States; Natalie L. Davis, University of Maryland School of Medicine, BALTIMORE, MD, United States

Shruti Patel, MD (she/her/hers)
Neonatology Fellow
University of Maryland School of Medicine
Baltimore, Maryland, United States
Presenting Author(s)
Background: Preterm infants fed breast milk have lower incidence of morbidities such as necrotizing enterocolitis (NEC), late onset sepsis, and bronchopulmonary dysplasia (BPD). Our urban level IV NICU therefore introduced human-milk-based human-milk fortifiers (HMBHMF) in November 2018 for neonates born < 1250g who were being fed maternal breast milk (MBM) or donor breast milk (DBM) to meet nutritional goals while providing an early exclusive human milk diet. However, HMBHMF is significantly more expensive than bovine-based fortifiers and concern exists about poorer somatic growth.
Objective: To evaluate the effect of HMBHMF introduction on NEC incidence, feeding characteristics, and growth during NICU admission.
Design/Methods: Retrospective medical record review of infants born < 1250g in 2 cohorts: 1) prior to introduction of HMBHMF with bovine-based HMF only (9/2015 - 10/2018) and 2) after introduction of HMBHMF (11/2018 - 12/2020). Compared clinical and demographic characteristics between those who received only bovine-based HMF vs. those receiving HMBHMF. Performed multivariable logistic regression to assess the association between HMBHMF use and NEC stage 2/3, controlling for changes in feeding protocols over time.
Results: We identified 419 subjects, of whom 121 (29%) received HMBHMF. Infants completed HMBHMF by median day of life 43 [IQR 31-56]. Neonatal characteristics were similar over time in regards to sex, birth weight, and race. [Table 1] Feeding with HMBHMF was not associated with lower incidence of NEC, BPD, retinopathy of prematurity requiring treatment, periventricular leukomalacia, intraventricular hemorrhages, late onset sepsis, or death. Introduction of HMBHMF did not affect time to full enteral feeds, time to completion of parenteral nutrition, need-for or duration-of central access. Infants fed HMBHMF had a slower weight gain (g/kg/day) at 4 weeks (p=0.0008) and 6 weeks (p=0.0493) compared to those prior to introduction of HMBHMF. Using a multivariable logistic regression model for predictors of stage 2 or 3 NEC, HMBHMF did not decrease the odds of NEC (p=0.9730). [Table 2] Lower birth gestational age and premature prolonged rupture of membranes were significantly associated with NEC.Conclusion(s): Infants fed HMBHMF had slower growth rates without improvement in feeding characteristics such as timing of full feeds and reduction in central line days. Despite the presumed potential for lowering the incidence of NEC with an early exclusive human milk diet, our study found no association between introduction of HMBHMF and reduction in rates of NEC or other common comorbidities of prematurity.
Table 1. Bivariate Comparison of Characteristics Before and After Introduction of HMBHMF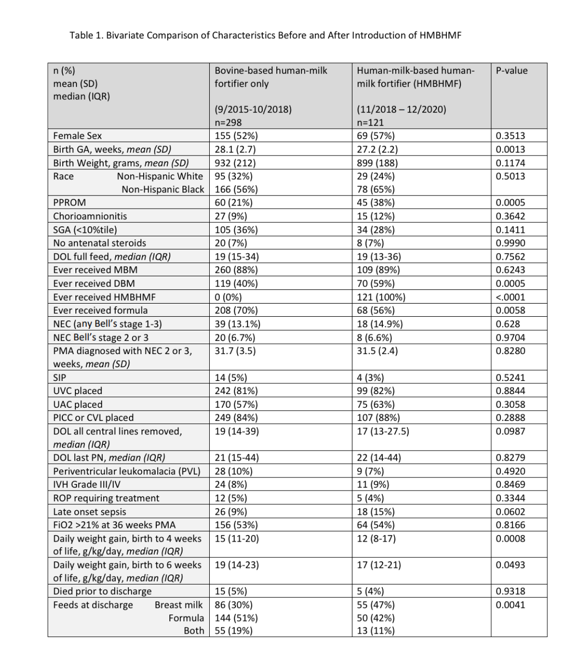 CVL, central venous line surgically placed; DBM, donor breast milk; DOL, day of life; GA, gestational age; HMBHMF, human-milk-based human-milk fortifier; IVH, intraventricular hemorrhage; MBM, maternal breast milk; NEC, necrotizing enterocolitis; PICC, peripherally inserted central catheter; PMA, post-menstrual age in weeks; PN, parenteral nutrition; PPROM, prolonged premature rupture of membranes; ROP, retinopathy of prematurity; SGA, small for gestational age status < 10%tile; SIP, spontaneous intestinal perforation; UAC, umbilical arterial catheter; UVC, umbilical venous catheter
CVL, central venous line surgically placed; DBM, donor breast milk; DOL, day of life; GA, gestational age; HMBHMF, human-milk-based human-milk fortifier; IVH, intraventricular hemorrhage; MBM, maternal breast milk; NEC, necrotizing enterocolitis; PICC, peripherally inserted central catheter; PMA, post-menstrual age in weeks; PN, parenteral nutrition; PPROM, prolonged premature rupture of membranes; ROP, retinopathy of prematurity; SGA, small for gestational age status < 10%tile; SIP, spontaneous intestinal perforation; UAC, umbilical arterial catheter; UVC, umbilical venous catheter
Table 2. Multivariable Logistic Regression Model of Predictors of Stage 2 or 3 NEC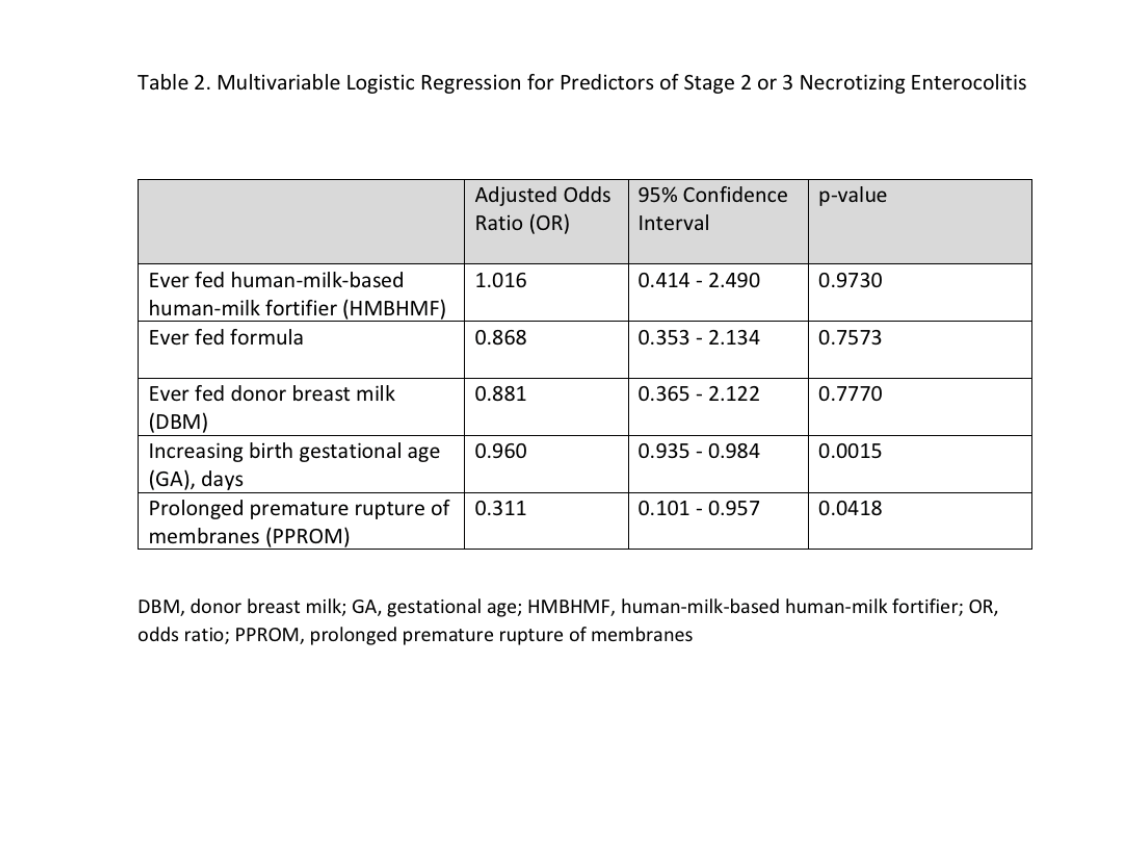 Values adjusted for all variables in the model.
Values adjusted for all variables in the model.
Objective: To evaluate the effect of HMBHMF introduction on NEC incidence, feeding characteristics, and growth during NICU admission.
Design/Methods: Retrospective medical record review of infants born < 1250g in 2 cohorts: 1) prior to introduction of HMBHMF with bovine-based HMF only (9/2015 - 10/2018) and 2) after introduction of HMBHMF (11/2018 - 12/2020). Compared clinical and demographic characteristics between those who received only bovine-based HMF vs. those receiving HMBHMF. Performed multivariable logistic regression to assess the association between HMBHMF use and NEC stage 2/3, controlling for changes in feeding protocols over time.
Results: We identified 419 subjects, of whom 121 (29%) received HMBHMF. Infants completed HMBHMF by median day of life 43 [IQR 31-56]. Neonatal characteristics were similar over time in regards to sex, birth weight, and race. [Table 1] Feeding with HMBHMF was not associated with lower incidence of NEC, BPD, retinopathy of prematurity requiring treatment, periventricular leukomalacia, intraventricular hemorrhages, late onset sepsis, or death. Introduction of HMBHMF did not affect time to full enteral feeds, time to completion of parenteral nutrition, need-for or duration-of central access. Infants fed HMBHMF had a slower weight gain (g/kg/day) at 4 weeks (p=0.0008) and 6 weeks (p=0.0493) compared to those prior to introduction of HMBHMF. Using a multivariable logistic regression model for predictors of stage 2 or 3 NEC, HMBHMF did not decrease the odds of NEC (p=0.9730). [Table 2] Lower birth gestational age and premature prolonged rupture of membranes were significantly associated with NEC.Conclusion(s): Infants fed HMBHMF had slower growth rates without improvement in feeding characteristics such as timing of full feeds and reduction in central line days. Despite the presumed potential for lowering the incidence of NEC with an early exclusive human milk diet, our study found no association between introduction of HMBHMF and reduction in rates of NEC or other common comorbidities of prematurity.
Table 1. Bivariate Comparison of Characteristics Before and After Introduction of HMBHMF
 CVL, central venous line surgically placed; DBM, donor breast milk; DOL, day of life; GA, gestational age; HMBHMF, human-milk-based human-milk fortifier; IVH, intraventricular hemorrhage; MBM, maternal breast milk; NEC, necrotizing enterocolitis; PICC, peripherally inserted central catheter; PMA, post-menstrual age in weeks; PN, parenteral nutrition; PPROM, prolonged premature rupture of membranes; ROP, retinopathy of prematurity; SGA, small for gestational age status < 10%tile; SIP, spontaneous intestinal perforation; UAC, umbilical arterial catheter; UVC, umbilical venous catheter
CVL, central venous line surgically placed; DBM, donor breast milk; DOL, day of life; GA, gestational age; HMBHMF, human-milk-based human-milk fortifier; IVH, intraventricular hemorrhage; MBM, maternal breast milk; NEC, necrotizing enterocolitis; PICC, peripherally inserted central catheter; PMA, post-menstrual age in weeks; PN, parenteral nutrition; PPROM, prolonged premature rupture of membranes; ROP, retinopathy of prematurity; SGA, small for gestational age status < 10%tile; SIP, spontaneous intestinal perforation; UAC, umbilical arterial catheter; UVC, umbilical venous catheterTable 2. Multivariable Logistic Regression Model of Predictors of Stage 2 or 3 NEC
 Values adjusted for all variables in the model.
Values adjusted for all variables in the model.