Neonatal GI Physiology & NEC
Category: Abstract Submission
Neonatal GI Physiology & NEC I
488 - Feasibility, Safety, and Short-Term Outcomes of Mucous Fistula Refeeding (MFR) in Infants with Intestinal failure (IF) and Stoma
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 488
Publication Number: 488.424
Publication Number: 488.424
Bianca E. Saenz, Baylor College of Medicine, Houston, TX, United States; Kimberly Fernandez Trahan, Baylor College of Medicine, Houston, TX, United States; Nidia B. Delgado, Texas Children's Hospital, Houston, TX, United States; Joseph Hagan, Baylor College of Medicine, Houston, TX, United States; Ganga Gokulakrishnan, Baylor College of Medicine, Houston, TX, United States; Muralidhar Premkumar, Baylor College of Medicine, Houston, TX, United States

Bianca E. Saenz, MD (she/her/hers)
Pediatric Nephrology Fellow
Baylor College of Medicine - Texas Childrens Hospital
Houston, Texas, United States
Presenting Author(s)
Background: Infants with intestinal failure (IF) requiring stoma and mucous fistula creation are faced with unique nutritional challenges in the period prior to reanastomosis (RA). Enteral nutrition (EN) advancement reduces risk of complications associated with parenteral nutrition (PN), such as blood stream infections and cholestasis, though this must be balanced with feed tolerance and optimal growth. It is unclear if mucous fistula refeeding (MFR) prior to RA is safe and beneficial in this population.
Objective: To investigate the feasibility, safety, and short-term outcomes of MFR in infants with IF and stoma prior to RA compared to similar infants who did not receive MFR.
Design/Methods: Infants with IF requiring stoma creation and RA admitted to Texas Children's Hospital between January 2014 and December 2020 were studied. The practice of MFR was adopted at Texas Children’s Hospital in 2018. Infants in No-MFR group (2014-2018) were compared to those who received MFR (2018-2020). Patient demographics, surgical characteristics, PN use, EN advancement, growth, and complications were compared.
Results: Of 68 patients included, 52 were(76.5%) in the No-MFR group and 16 (23.5%) in the MFR group. Patient characteristics were similar except for the occurrence of NEC in the No-MFR group (Table1). Outcomes of patients in the MFR and No-MFR groups are compared in Table 2. In the MFR group, 8/16 (50%) infants achieved enteral autonomy (EA) prior to RA compared to 14/52 (27%) in the No-MFR group (p=0.126). Due to poor growth, PN was restarted in 5/8 (63%) infants in MFR group who had achieved EA prior to RA. Median maximum feed volume prior to RA was 90 ml/kg/d in No-MFR group vs. 110 ml/kg/d in MFR group (p=0.331). Peak conjugated bilirubin (CB) trended lower in MFR group, but did not reach significance (4.5 vs. 2.4 mg/dL; p=0.107). PN days after RA, anthropometrics at RA and discharge, and length of hospitalization were not different between groups.Conclusion(s): In this small retrospective study of infants with IF and stoma, MFR demonstrated to be feasible and safe. Infants with MFR achieved similar short-term outcomes including growth at discharge compared to infants who did not receive MFR. The MFR group had higher EA and enteral feed volumes and lower CB levels of clinical significance prior to RA. Though likely due to smaller sample size, this did not meet statistical significance. Achieving enteral autonomy prior to RA with MFR is possible but challenging due to difficulties in achieving optimal growth without PN support. MFR in infants with IF and stoma is safe with tangible benefits and warrants further study.
Table 1. Comparison of the characteristics of patients in the MFR versus no MFR groups.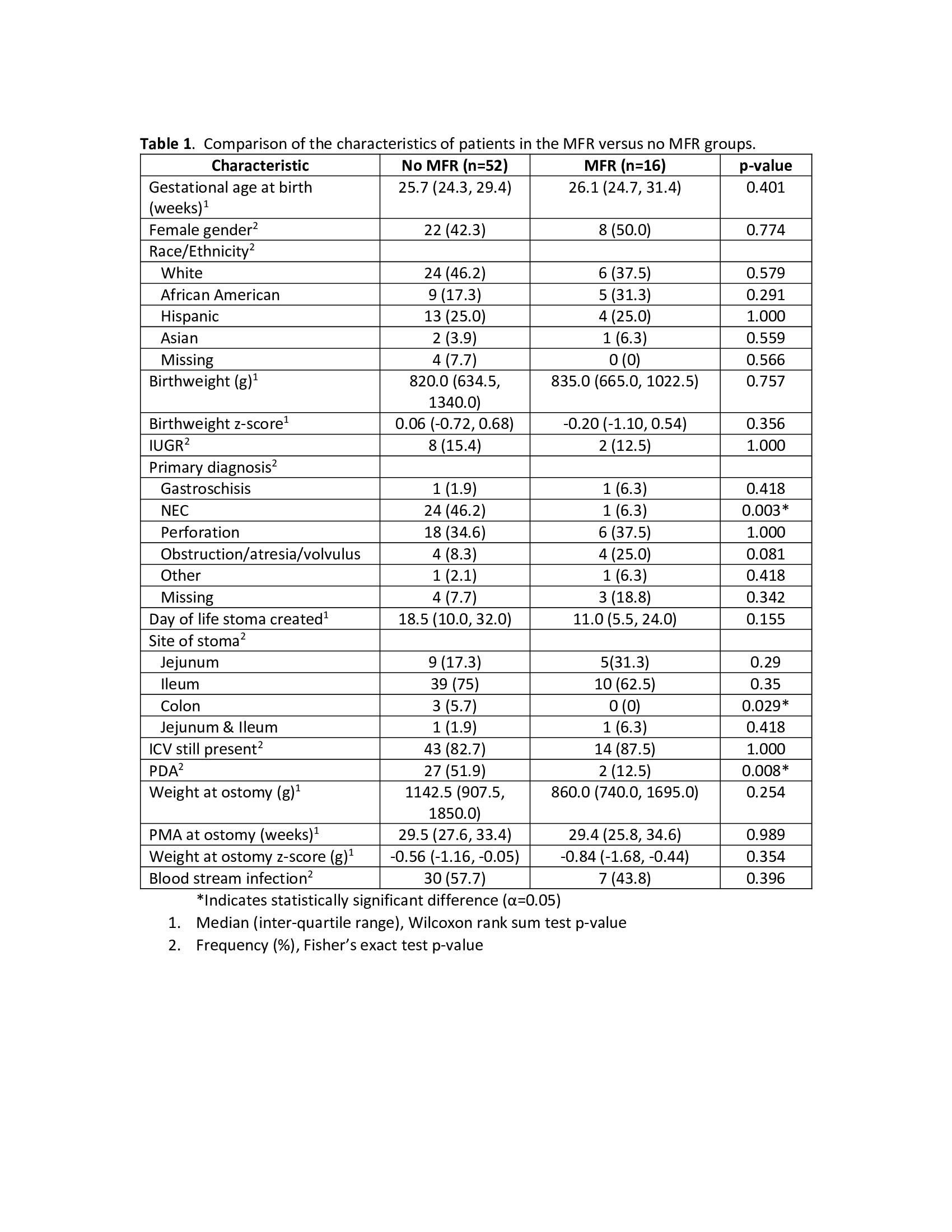
Table 2. Comparison of the outcomes of patients in the MFR versus no MFR groups.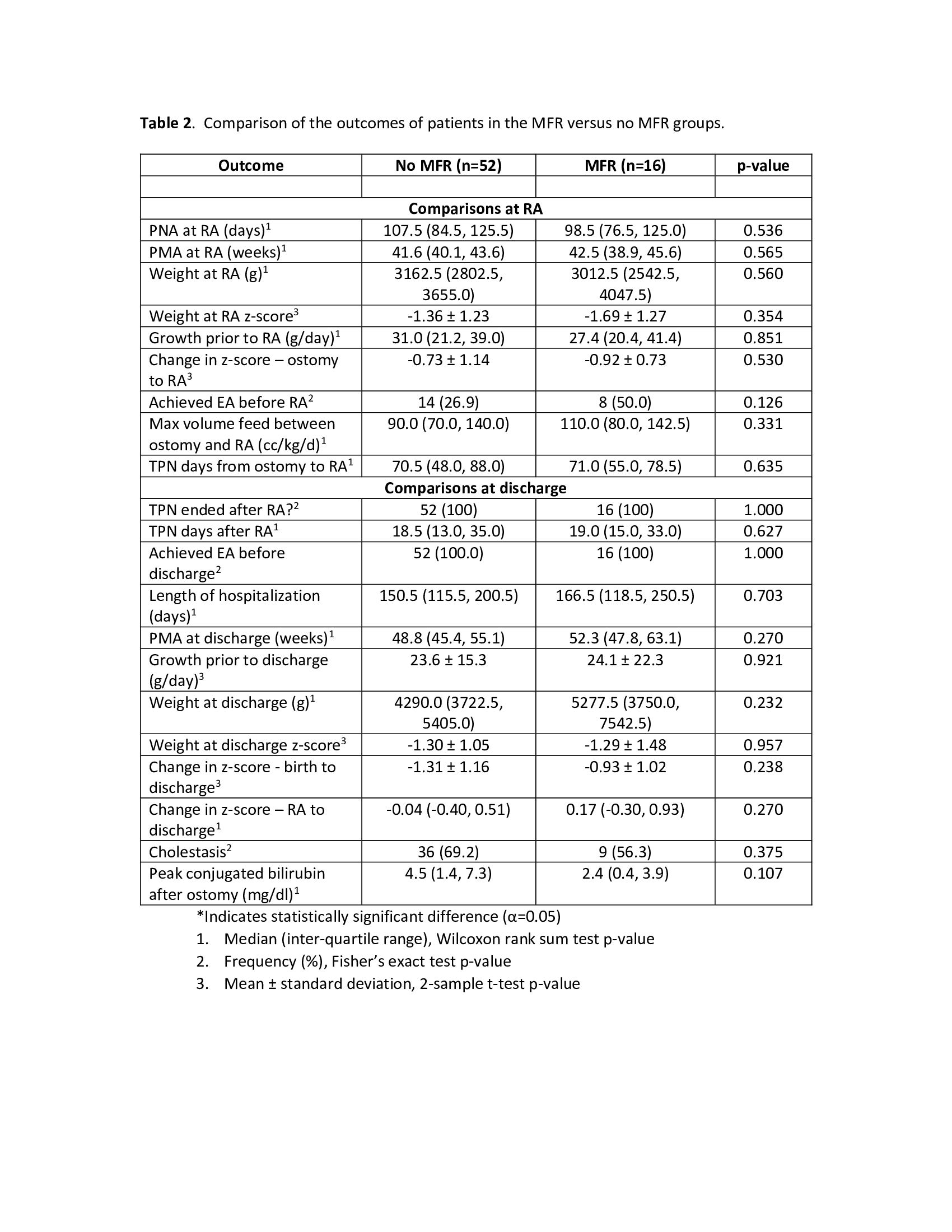
Objective: To investigate the feasibility, safety, and short-term outcomes of MFR in infants with IF and stoma prior to RA compared to similar infants who did not receive MFR.
Design/Methods: Infants with IF requiring stoma creation and RA admitted to Texas Children's Hospital between January 2014 and December 2020 were studied. The practice of MFR was adopted at Texas Children’s Hospital in 2018. Infants in No-MFR group (2014-2018) were compared to those who received MFR (2018-2020). Patient demographics, surgical characteristics, PN use, EN advancement, growth, and complications were compared.
Results: Of 68 patients included, 52 were(76.5%) in the No-MFR group and 16 (23.5%) in the MFR group. Patient characteristics were similar except for the occurrence of NEC in the No-MFR group (Table1). Outcomes of patients in the MFR and No-MFR groups are compared in Table 2. In the MFR group, 8/16 (50%) infants achieved enteral autonomy (EA) prior to RA compared to 14/52 (27%) in the No-MFR group (p=0.126). Due to poor growth, PN was restarted in 5/8 (63%) infants in MFR group who had achieved EA prior to RA. Median maximum feed volume prior to RA was 90 ml/kg/d in No-MFR group vs. 110 ml/kg/d in MFR group (p=0.331). Peak conjugated bilirubin (CB) trended lower in MFR group, but did not reach significance (4.5 vs. 2.4 mg/dL; p=0.107). PN days after RA, anthropometrics at RA and discharge, and length of hospitalization were not different between groups.Conclusion(s): In this small retrospective study of infants with IF and stoma, MFR demonstrated to be feasible and safe. Infants with MFR achieved similar short-term outcomes including growth at discharge compared to infants who did not receive MFR. The MFR group had higher EA and enteral feed volumes and lower CB levels of clinical significance prior to RA. Though likely due to smaller sample size, this did not meet statistical significance. Achieving enteral autonomy prior to RA with MFR is possible but challenging due to difficulties in achieving optimal growth without PN support. MFR in infants with IF and stoma is safe with tangible benefits and warrants further study.
Table 1. Comparison of the characteristics of patients in the MFR versus no MFR groups.

Table 2. Comparison of the outcomes of patients in the MFR versus no MFR groups.

