Pediatric Nutrition
Category: Abstract Submission
Pediatric Nutrition I
249 - GI Symptom Improvement in Infants with Suspected CMPA Treated with Extensively Hydrolyzed Formula in a Clinical Setting: A Prospective Cohort Analysis
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 249
Publication Number: 249.141
Publication Number: 249.141
Michael J. Wilsey, USF Health Morsani College of Medicine, St. Petersburg, FL, United States; Lea V. Oliveros, Johns Hopkins All Children's Hospital, Saint Petersburg, FL, United States; Kathryn M. Kimsey, Johns Hopkins All Children's Hospital, Tampa, FL, United States; Grafton S. Barnett, Johns Hopkins All Children's Hospital, Harvest, AL, United States; Jerry M. Brown, Johns Hopkins All Children's Hospital, New port Richey, FL, United States

Jerry M. Brown, II
Student Researcher
Johns Hopkins All Children's Hospital
New port Richey, Florida, United States
Presenting Author(s)
Background: Cow’s milk protein allergy (CMPA) occurs in 2-3% in infants. Long-term efficacy of extensively hydrolyzed formulas (EHF) in treating CPMA is well established. Parents and healthcare providers (HCP) prioritize rapid reduction of CMPA symptoms in the short-term as the main treatment goal. Data regarding short-term efficacy of treating infants with CMPA with EHF is limited.
Objective: We sought to determine the short-term efficacy of gastrointestinal (GI) symptom relief in infants < 6 months of age with suspected CMPA treated with EHF containing the probiotic Lactobacillus rhamnosus GG (EHF-LGG), a probiotic previously shown to be efficacious in augmenting the clinical response of an extensively hydrolyzed formula in infants with CMPA.
Design/Methods: This is a prospective cohort analysis of de-identified survey data obtained from healthcare providers (HCP) treating 222 patients < 6 months of age with symptoms consistent with CMPA. Demographic data and baseline symptoms were collected via ZS Moments, a mobile app on an electronic device, by HCP. GI CMPA symptoms scored by severity from 0 to 3 (none, low, moderate, severe) at the first visit and were then started on an extensively hydrolyzed formula. Patients were reevaluated at follow-up to assess for changes in symptom severity and symptoms scoring was repeated.
Results: Results are summarized in Table 1. Most HCP's (87%) involved in the survey were general pediatricians. Of 222 patients identified, 202 (91%) were included for symptom analysis. 55% were male and 59% had a family history of atopy. Most (~3/4) were newly diagnosed with CMPA, the majority (93%) were diagnosed by clinical assessment, and ~2/3 were seen in follow-up within 3 to 5 weeks. Symptom improvement was consistent across different follow-up visit durations between visit 1 and 2. The most common symptoms were diarrhea (n=117; 58%), regurgitation (n=142; 70%), abdominal pain (n=105, 52%), and belching (n=118, 58%). Of those with GI symptoms at visit 1, 93% (n=193) improved at visit 2.Conclusion(s): This is the largest prospective survey in the US examining short-term efficacy of GI symptom reduction using EHF-LGG in infants < 6 months with CMP. Analysis of this prospective cohort showed significant improvement in GI symptoms of CMPA in infants under 6 months of age when treated with extensively hydrolyzed formula. EHF-LGG appears to provide clinical relief and rapid reduction of GI symptoms in infants < 6 months of age with symptoms consistent with CMPA, often by the next follow-up visit.
Percentage of Patients Reporting Improvement of GI Symptoms at Visit 2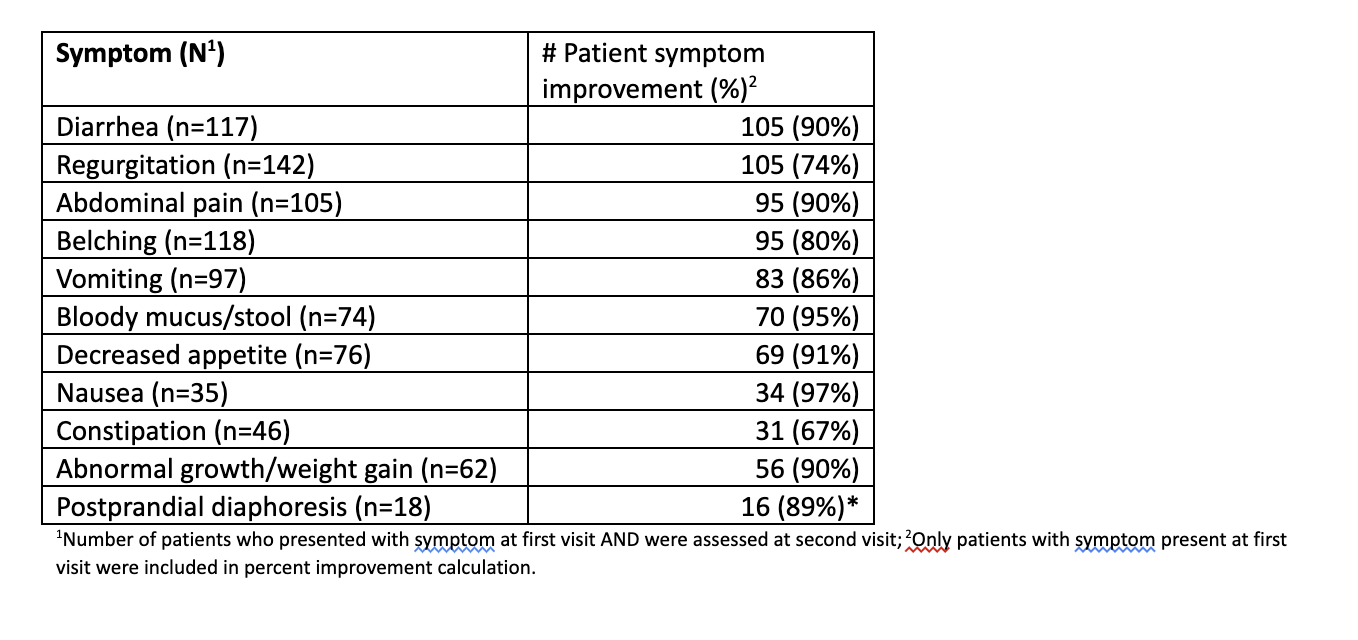
Objective: We sought to determine the short-term efficacy of gastrointestinal (GI) symptom relief in infants < 6 months of age with suspected CMPA treated with EHF containing the probiotic Lactobacillus rhamnosus GG (EHF-LGG), a probiotic previously shown to be efficacious in augmenting the clinical response of an extensively hydrolyzed formula in infants with CMPA.
Design/Methods: This is a prospective cohort analysis of de-identified survey data obtained from healthcare providers (HCP) treating 222 patients < 6 months of age with symptoms consistent with CMPA. Demographic data and baseline symptoms were collected via ZS Moments, a mobile app on an electronic device, by HCP. GI CMPA symptoms scored by severity from 0 to 3 (none, low, moderate, severe) at the first visit and were then started on an extensively hydrolyzed formula. Patients were reevaluated at follow-up to assess for changes in symptom severity and symptoms scoring was repeated.
Results: Results are summarized in Table 1. Most HCP's (87%) involved in the survey were general pediatricians. Of 222 patients identified, 202 (91%) were included for symptom analysis. 55% were male and 59% had a family history of atopy. Most (~3/4) were newly diagnosed with CMPA, the majority (93%) were diagnosed by clinical assessment, and ~2/3 were seen in follow-up within 3 to 5 weeks. Symptom improvement was consistent across different follow-up visit durations between visit 1 and 2. The most common symptoms were diarrhea (n=117; 58%), regurgitation (n=142; 70%), abdominal pain (n=105, 52%), and belching (n=118, 58%). Of those with GI symptoms at visit 1, 93% (n=193) improved at visit 2.Conclusion(s): This is the largest prospective survey in the US examining short-term efficacy of GI symptom reduction using EHF-LGG in infants < 6 months with CMP. Analysis of this prospective cohort showed significant improvement in GI symptoms of CMPA in infants under 6 months of age when treated with extensively hydrolyzed formula. EHF-LGG appears to provide clinical relief and rapid reduction of GI symptoms in infants < 6 months of age with symptoms consistent with CMPA, often by the next follow-up visit.
Percentage of Patients Reporting Improvement of GI Symptoms at Visit 2

