Neonatal Neurology: Translational
Category: Abstract Submission
Neurology 6: Neonatal Neurology Preterm Imaging
339 - Objectively Diagnosed Diffuse Excessive High Signal Intensity (DEHSI) in the Very Preterm Brain Represents Reduced Branching and Cellularity of the Major Sensorimotor Pathways
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 339
Publication Number: 339.442
Publication Number: 339.442
Julia Kline, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Venkata Sita Priyanka Illapani, Children's National Hospital, Falls Church, VA, United States; Hailong Li, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Lili He, Cincinnati Children's Hospital, Cincinnati, OH, United States; Jonathan Dudley, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Jean A. Tkach, CCHMC, Cincinnati, OH, United States; Weihong Yuan, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States; Nehal Parikh, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States

Nehal A. Parikh, DO, MS
Professor of Pediatrics
Cincinnati Children's Hospital Medical Center
Cincinnati, Ohio, United States
Presenting Author(s)
Background: In 50-80% of preterm brains, a hyperintense signal—DEHSI—appears on T2-w MRI at term-equivalent age, prominently in the centrum semiovale (CS) and periventricular white matter (WM). Some that have objectively quantified DEHSI (referred to as diffuse white matter abnormality [DWMA]) have shown a significant association with neurodevelopmental deficits. Abnormal diffusion properties, altered resting state connectivity, fewer axons/oligodendrocytes, and decreased network efficiency have been documented in small studies of DWMA, indicating sparse/disorganized WM. We leveraged newer diffusion MRI models, Constrained Spherical Deconvolution (CSD) and Neurite Orientation Dispersion and Density Imaging (NODDI), to better characterize the WM microstructure of DWMA.
Objective: We hypothesized that with increasing DWMA volume: 1) orientation dispersion index (ODI), neurite density index (NDI), and fractional anisotropy (FA) from the NODDI model would each decrease and 2) fiber density (FD), fiber cross section (FC), and their product (FDC) from the CSD model would also decrease. CSD and NODDI outcomes were analyzed with connectivity-based fixel enhancement and Tract-Based Spatial Statistics, respectively.
Design/Methods: In a prospective cohort of 265 very preterm infants, we quantified DWMA on T2 MRI using our deep learning algorithm. We corrected for age at MRI, gestational age, birth weight, bronchopulmonary dysplasia, retinopathy of prematurity, oxygen therapy, postnatal steroids—variables that differed between the highest/lowest DWMA groups (determined using quartiles)—and Kidokoro’s global brain abnormality score.
Results: 202 subjects had quantified DWMA and passed QA for both models. With increasing DWMA: 1) FD and FDC decreased throughout the WM, and FC decreased in the major sensorimotor tracts traversing the CS and cerebellum (Fig. 1) and 2) NDI and FA decreased throughout the WM and ODI decreased in the CS and parts of the corona radiata/temporal lobe (Fig. 2). DWMA was associated with widespread WM abnormalities: reduced axonal density (NDI/ FD), reduced axonal fanning/branching (ODI) in the CS—a critical region for information transfer—and reduced thickness (FC) of the corticospinal tracts. A brain with fewer neurons may prioritize reduced branching/complexity, to keep core motor functions intact, while sacrificing accessory motor functions. This may explain the prevalence of cerebral palsy/sensorimotor disorders in preterm populations, who tend to have DWMA. Conclusion(s): These findings provide insight into DWMA’s biological underpinnings and demonstrate that it is a serious pathology.
Figure 1. Streamlines in which FD/FC/FDC were negatively associated with DWMA extent. The color bar indicates the absolute effect size.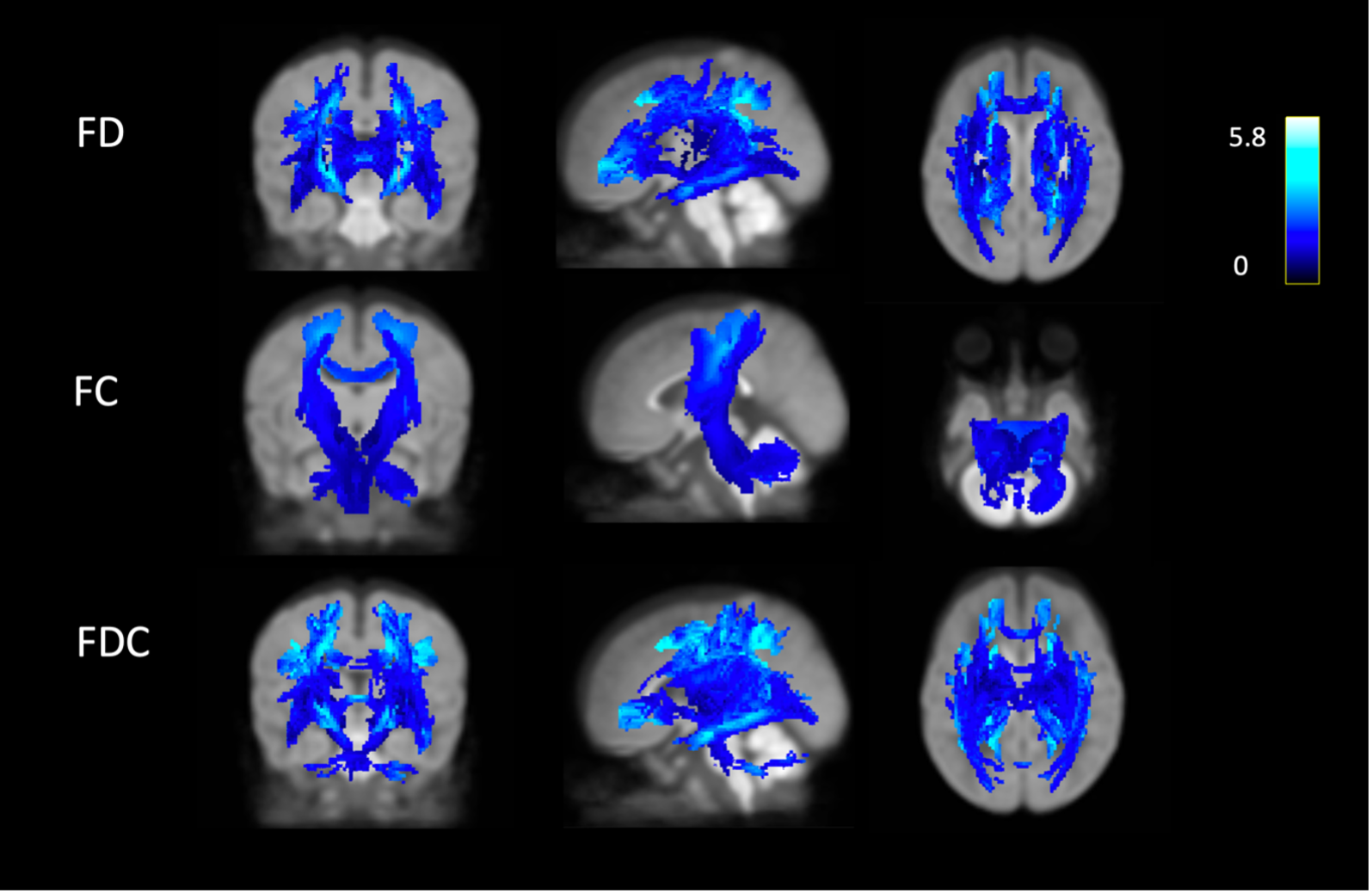
TBSS results showing WM regions in which FA/ODI/NDI from the NODDI model decreased with DWMA extent. Green areas indicate the TBSS WM skeleton.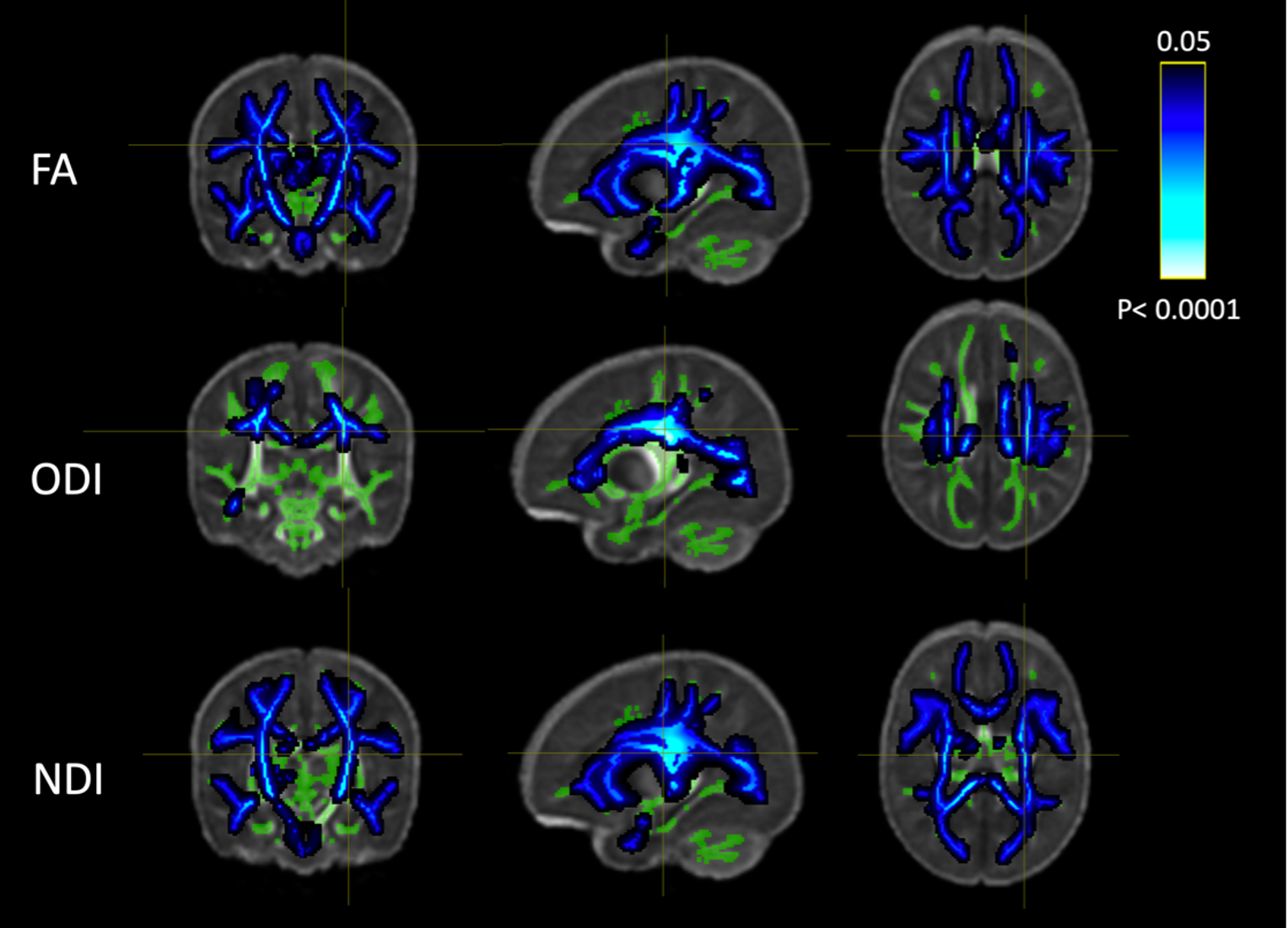
Objective: We hypothesized that with increasing DWMA volume: 1) orientation dispersion index (ODI), neurite density index (NDI), and fractional anisotropy (FA) from the NODDI model would each decrease and 2) fiber density (FD), fiber cross section (FC), and their product (FDC) from the CSD model would also decrease. CSD and NODDI outcomes were analyzed with connectivity-based fixel enhancement and Tract-Based Spatial Statistics, respectively.
Design/Methods: In a prospective cohort of 265 very preterm infants, we quantified DWMA on T2 MRI using our deep learning algorithm. We corrected for age at MRI, gestational age, birth weight, bronchopulmonary dysplasia, retinopathy of prematurity, oxygen therapy, postnatal steroids—variables that differed between the highest/lowest DWMA groups (determined using quartiles)—and Kidokoro’s global brain abnormality score.
Results: 202 subjects had quantified DWMA and passed QA for both models. With increasing DWMA: 1) FD and FDC decreased throughout the WM, and FC decreased in the major sensorimotor tracts traversing the CS and cerebellum (Fig. 1) and 2) NDI and FA decreased throughout the WM and ODI decreased in the CS and parts of the corona radiata/temporal lobe (Fig. 2). DWMA was associated with widespread WM abnormalities: reduced axonal density (NDI/ FD), reduced axonal fanning/branching (ODI) in the CS—a critical region for information transfer—and reduced thickness (FC) of the corticospinal tracts. A brain with fewer neurons may prioritize reduced branching/complexity, to keep core motor functions intact, while sacrificing accessory motor functions. This may explain the prevalence of cerebral palsy/sensorimotor disorders in preterm populations, who tend to have DWMA. Conclusion(s): These findings provide insight into DWMA’s biological underpinnings and demonstrate that it is a serious pathology.
Figure 1. Streamlines in which FD/FC/FDC were negatively associated with DWMA extent. The color bar indicates the absolute effect size.

TBSS results showing WM regions in which FA/ODI/NDI from the NODDI model decreased with DWMA extent. Green areas indicate the TBSS WM skeleton.

