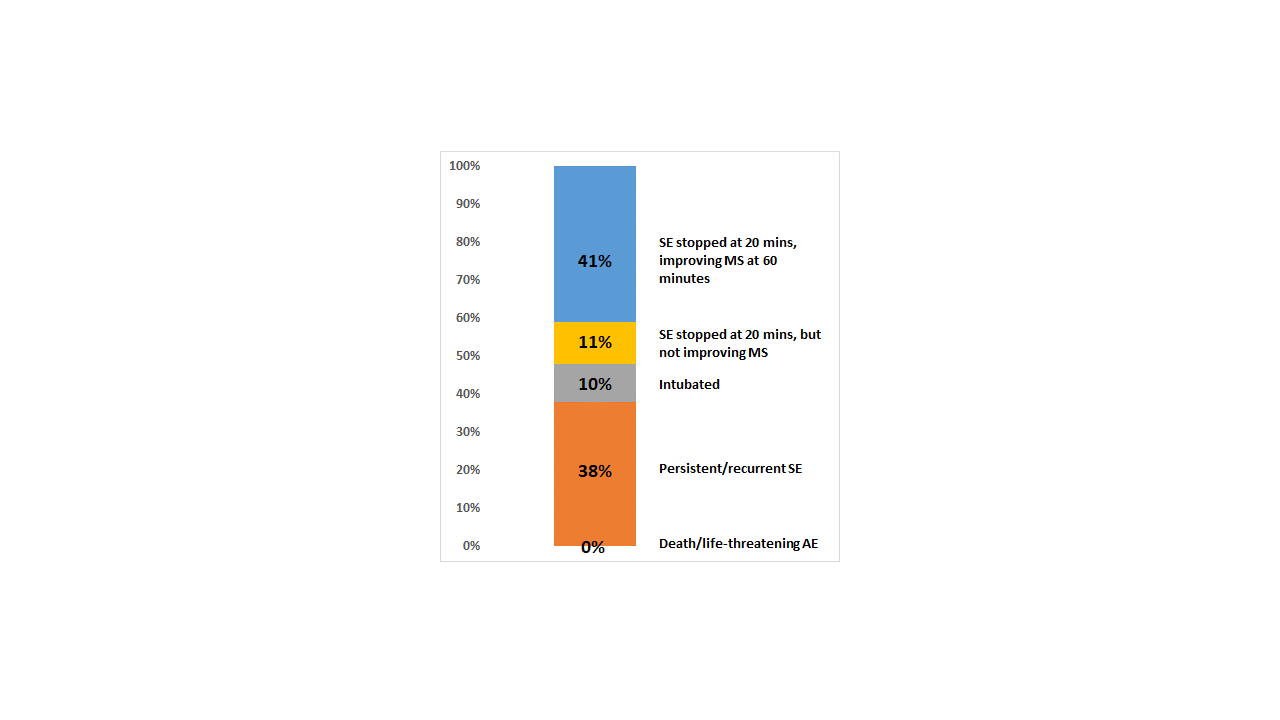Emergency Medicine: All Areas
Category: Abstract Submission
Emergency Medicine XII
18 - DOOR-STEP: A Desirability of Outcome Ranking Scale for Clinical Trials of Status Epilepticus Treatment
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 18
Publication Number: 18.405
Publication Number: 18.405
James Chamberlain, Children's National Health System, Washington, DC, United States; Jaideep Kapur, University of Virginia School of Medicine, Charlottesville, VA, United States; Robert Silbergleit, University of Michigan Medical School, Ann Arbor, MI, United States; Jordan J. Elm, Medical University of South Carolina College of Medicine, Charleston, SC, United States; Thomas P. Bleck, Northwestern University Feinberg School of Medicine, Chicago, IL, United States; Shlomo Shinnar, Montefiore Medical Center, Albert Einstein College of Medicine, Bronx, NY, United States; Shahriar Zehtabchi, Department of Emergency Medicine, Downstate Health Sciences University, Brooklyn, NY, United States; Scott Evans, Milken Institute School of Public Health, Potomac, MD, United States

James Chamberlain, MD (he/him/his)
Professor
Children's National Health System
Washington, District of Columbia, United States
Presenting Author(s)
Background: Previous clinical trials of status epilepticus (SE) treatment have used an approach separating binary success/non-success efficacy outcomes from safety outcomes. This approach fails to account for intermediate clinical outcomes and the balancing of harm and benefit inherent in the treatment of SE.
Objective: 1. To develop a composite benefit-harm outcome measure for future studies of SE using the DOOR method.
2. To apply the composite outcome measure to patients in a prior trial of SE, the Established SE Treatment Trial (ESETT)
Design/Methods: We convened an expert panel of epileptologists, clinical neurophysiologists, neurocritical care physicians, and emergency physicians to determine a clinical continuum of desirable outcomes for SE. Experts anchored on a perfect outcome (resolved SE and improving mental status) and the worst possible outcome (life-threatening cardiac event or death) and determined intermediate clinical outcomes of graded desirability. Discussion occurred until consensus was achieved.
Results: For a drug intervention infused over 5 minutes, with an initial expected pharmacodynamic effect by 15 minutes, we constructed a five-point Desirability of Outcome Ranking scale (Figure 1), consisting of the following outcomes (ranked best-to-worst):
1.No clinically evident or electrographic SE occurring after 15 minutes, not intubated, and mental status improving by 60 minutes.
2.No clinical or electrographic evidence of SE after 15 minutes, not intubated, but mental status not improving at 60 minutes.
3.No clinical or electrographic evidence of SE, AND either intubation OR the use of rescue medications.
4.Any clinical or electrographic evidence of SE after 15 minutes (i.e. seizures for 5 consecutive minutes or 9 cumulative minutes [20% seizure burden by time] or seizures persisting until administration of rescue medication).
5.Life-threatening adverse event or death.
Figure 2 depicts a retrospective hypothetical application of this method to the outcomes
of the levetiracetam (LEV) arm of the Established Status Epilepticus Treatment Trial (in
which outcomes were binary). Conclusion(s): We propose a graded Desirability of Outcome Ranking scale for clinical trials of SE (DOOR-STEP). The scale is intuitive, has strong face validity, and allows for a balanced measurement of safety and efficacy as a single composite endpoint.
Figure 1. Desirability of Outcomes Ranking for a trial of status epilepticus (DOOR-STEP)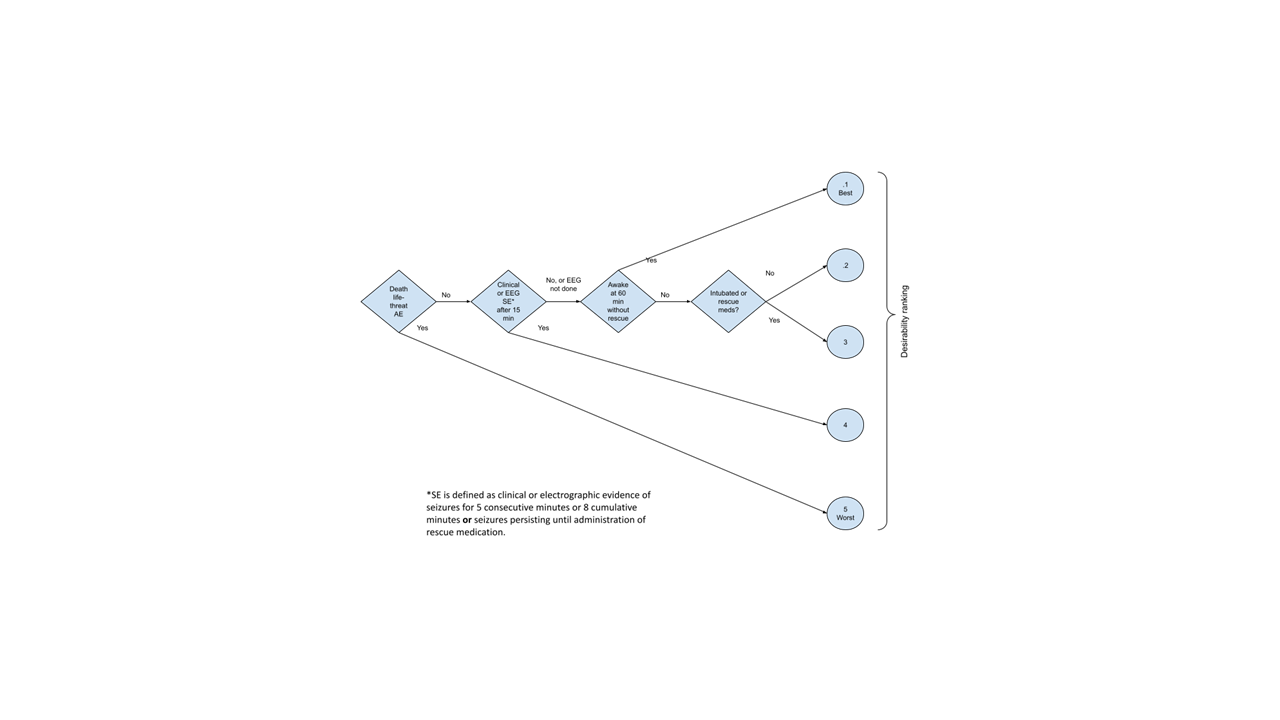
Figure 2. DOOR-STEP applied retrospectively to a prior clinical trial of status epilepticus (ESETT), in which outcomes were binary.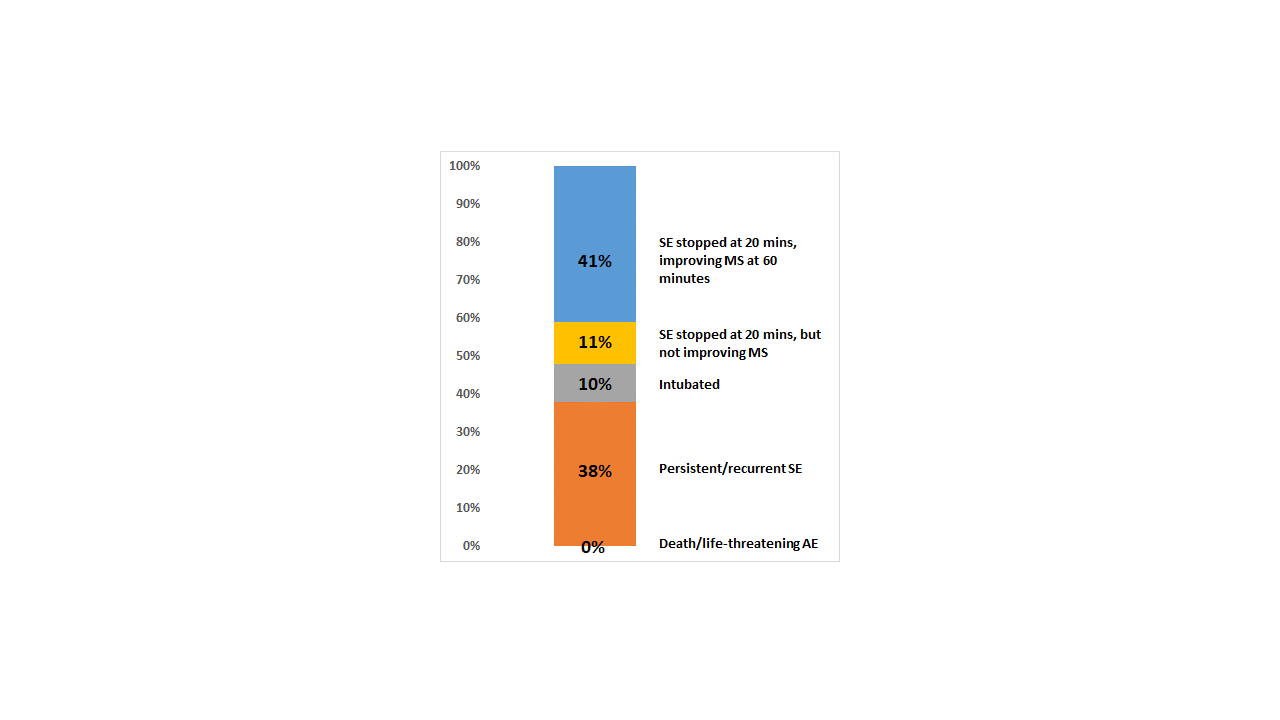
Objective: 1. To develop a composite benefit-harm outcome measure for future studies of SE using the DOOR method.
2. To apply the composite outcome measure to patients in a prior trial of SE, the Established SE Treatment Trial (ESETT)
Design/Methods: We convened an expert panel of epileptologists, clinical neurophysiologists, neurocritical care physicians, and emergency physicians to determine a clinical continuum of desirable outcomes for SE. Experts anchored on a perfect outcome (resolved SE and improving mental status) and the worst possible outcome (life-threatening cardiac event or death) and determined intermediate clinical outcomes of graded desirability. Discussion occurred until consensus was achieved.
Results: For a drug intervention infused over 5 minutes, with an initial expected pharmacodynamic effect by 15 minutes, we constructed a five-point Desirability of Outcome Ranking scale (Figure 1), consisting of the following outcomes (ranked best-to-worst):
1.No clinically evident or electrographic SE occurring after 15 minutes, not intubated, and mental status improving by 60 minutes.
2.No clinical or electrographic evidence of SE after 15 minutes, not intubated, but mental status not improving at 60 minutes.
3.No clinical or electrographic evidence of SE, AND either intubation OR the use of rescue medications.
4.Any clinical or electrographic evidence of SE after 15 minutes (i.e. seizures for 5 consecutive minutes or 9 cumulative minutes [20% seizure burden by time] or seizures persisting until administration of rescue medication).
5.Life-threatening adverse event or death.
Figure 2 depicts a retrospective hypothetical application of this method to the outcomes
of the levetiracetam (LEV) arm of the Established Status Epilepticus Treatment Trial (in
which outcomes were binary). Conclusion(s): We propose a graded Desirability of Outcome Ranking scale for clinical trials of SE (DOOR-STEP). The scale is intuitive, has strong face validity, and allows for a balanced measurement of safety and efficacy as a single composite endpoint.
Figure 1. Desirability of Outcomes Ranking for a trial of status epilepticus (DOOR-STEP)

Figure 2. DOOR-STEP applied retrospectively to a prior clinical trial of status epilepticus (ESETT), in which outcomes were binary.