Neonatal Quality Improvement
Category: Abstract Submission
Neonatal Quality Improvement III: GI and Nutrition
240 - Quality Improvement Project to Achieve Early Full Enteral Feeds in Preterm Infants
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 240
Publication Number: 240.128
Publication Number: 240.128
Usha Prasad, University of Connecticut School of Medicine, Hartford, CT, United States; Kendall Johnson, Connecticut Children's Medical Center, Hartford, CT, United States; McGuire McGuire, CT Childrens' Medical Center, Hartford, CT, United States; Kathleen Haines, Connecticut Children's, Hartford, CT, United States; AnnMarie Spizzoucco, Connecticut Children's Medical Center, Hartford, CT, United States; Shabnam Lainwala, Connecticut Children's Medical Center, Hartford, CT, United States
- UP
Usha Prasad, DO, MPH
Neonatal Perinatal Fellow
University of Connecticut School of Medicine
Hartford, Connecticut, United States
Presenting Author(s)
Background: Recent studies in preterm infants support early achievement of full enteral feeds to be associated with reduction in central line and total parental nutrition (TPN) days without adverse feeding outcomes.
Objective: The aim of this quality improvement (QI) project was to achieve full enteral feeds 20% sooner in preterm infants at a Level IV Neonatal Intensive Care Unit (NICU) by December 2021.
Design/Methods: Key drivers identified by a multidisciplinary team included obtain donor human milk (DHM) consent within 9 hours of life (HOL); initiate trophic feeds within 12 HOL; and modify enteral feeding guideline. Inborn infants with BW < 1.8 kg were included. Infants with congenital anomalies, transferred out prior to achievement of full enteral feeds or expired prior to initiation of enteral feeds were excluded. Baseline data from 5/1/2020 to 11/23/2020 and prospective data from 11/24/2020 to 10/31/2021 were collected.
The outcome measures were time to achieve full enteral feeds, central line and TPN days. The process measures were time in hours to obtain DHM consent and initiation of trophic feeds by 12 HOL. The balancing measures included DHM not used in infants where consent was obtained, incidence of feeds switched from bolus to continuous gastric feeds and the number of abdominal x-rays obtained within the first 30 days.
Results: 159 infants were included; 58 in baseline and 101 in post-intervention group. 16 infants were excluded.
Special cause variation (SCV) was detected for DHM consent time and time to full enteral feeds (Figures 1 and 2). There was a 23% improvement in achievement of full enteral feeds from 13.3 days to 10.3 days post-intervention. DHM consent time improved from 7 hours prior to birth (HPB) to 35 HPB post-intervention. Although SCV was not detected, a 21% improvement in central line days from 16.3 days to 12.9 days post-intervention and a 44% improvement in trophic feeds initiation from 32 HOL to 18 HOL was noted. No improvement in TPN days was seen. DHM consents obtained but not used decreased from 19% (11/58) to 4% (4/101) and continuous gastric feeds increased from 34% (20/58) to 39% (39/101) post-intervention. Number of abdominal x-rays obtained increased from 3 to 3.3 post-intervention.Conclusion(s): Through optimization of DHM consents, early trophic feed initiation and modification of feeding guideline, we achieved earlier time to full enteral feeds. Our next interventions will focus on improvement in TPN days while monitoring our balancing measures.
CVRevised CV_9.8.21.pdf
Figure 2. Days to Full Enteral Feeds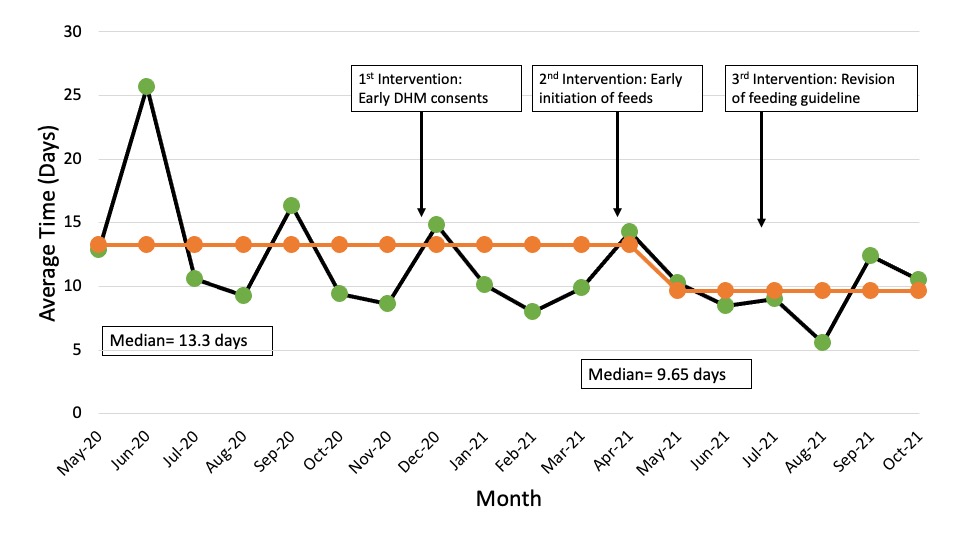
Objective: The aim of this quality improvement (QI) project was to achieve full enteral feeds 20% sooner in preterm infants at a Level IV Neonatal Intensive Care Unit (NICU) by December 2021.
Design/Methods: Key drivers identified by a multidisciplinary team included obtain donor human milk (DHM) consent within 9 hours of life (HOL); initiate trophic feeds within 12 HOL; and modify enteral feeding guideline. Inborn infants with BW < 1.8 kg were included. Infants with congenital anomalies, transferred out prior to achievement of full enteral feeds or expired prior to initiation of enteral feeds were excluded. Baseline data from 5/1/2020 to 11/23/2020 and prospective data from 11/24/2020 to 10/31/2021 were collected.
The outcome measures were time to achieve full enteral feeds, central line and TPN days. The process measures were time in hours to obtain DHM consent and initiation of trophic feeds by 12 HOL. The balancing measures included DHM not used in infants where consent was obtained, incidence of feeds switched from bolus to continuous gastric feeds and the number of abdominal x-rays obtained within the first 30 days.
Results: 159 infants were included; 58 in baseline and 101 in post-intervention group. 16 infants were excluded.
Special cause variation (SCV) was detected for DHM consent time and time to full enteral feeds (Figures 1 and 2). There was a 23% improvement in achievement of full enteral feeds from 13.3 days to 10.3 days post-intervention. DHM consent time improved from 7 hours prior to birth (HPB) to 35 HPB post-intervention. Although SCV was not detected, a 21% improvement in central line days from 16.3 days to 12.9 days post-intervention and a 44% improvement in trophic feeds initiation from 32 HOL to 18 HOL was noted. No improvement in TPN days was seen. DHM consents obtained but not used decreased from 19% (11/58) to 4% (4/101) and continuous gastric feeds increased from 34% (20/58) to 39% (39/101) post-intervention. Number of abdominal x-rays obtained increased from 3 to 3.3 post-intervention.Conclusion(s): Through optimization of DHM consents, early trophic feed initiation and modification of feeding guideline, we achieved earlier time to full enteral feeds. Our next interventions will focus on improvement in TPN days while monitoring our balancing measures.
CVRevised CV_9.8.21.pdf
Figure 2. Days to Full Enteral Feeds

