Endocrinology: Transgender
Category: Abstract Submission
Endocrinology I
527 - Rate of Polycythemia in Female-to-male Adolescent Transgender and Gender Non-conforming Patients on Testosterone Therapy
Friday, April 22, 2022
6:15 PM - 8:45 PM US MT
Poster Number: 527
Publication Number: 527.107
Publication Number: 527.107
Brit M. Foster, NemoursAlfred I. duPont Hospital for Children, Wilmington, DE, United States; Claire Loiselle, NemoursAlfred I. duPont Hospital for Children, Wilmington, DE, United States; Ran Zhang, Nemours Children's Hospital, Wilmington, DE, United States; Evan Graber, NemoursAlfred I. duPont Hospital for Children, Wilmington, DE, United States; MATTHEW D. DI GUGLIELMO, Sidney Kimmel Medical College at Thomas Jefferson University, WILMINGTON, DE, United States

Brit M. Foster, DO
Academic General Pediatric Fellow
NemoursAlfred I. duPont Hospital for Children
Wilmington, Delaware, United States
Presenting Author(s)
Background: Limited data exist on the biochemical impact of testosterone treatment in adolescent transgender male or gender non-conforming patients. Risks of suicidal ideation and suicide attempts are known to be increased in nontreated patients with gender dysphoria. However, gender affirming hormone therapy (GAHT) carries its own risks. Risk/benefit discussions are predominantly based on data from transgender adults or hypogonadal cisgender men. In adults undergoing testosterone therapy, polycythemia has been observed at a rate as high as 25%.
Objective: The study aim was to determine the rate of polycythemia for adolescent transgender male on GAHT with testosterone, clinical effects on this population, and risk of thrombosis.
Design/Methods: Patients seen in a Gender Wellness Program from 2015-2021 were studied. Included patients were started on testosterone therapy and data including hemoglobin (Hgb), hematocrit (Hct), and total serum testosterone (T) were collected at baseline and longitudinally. Data were analyzed using descriptive statistics and a linear mixed effect model.
Results: Of 189 patients, 104 were treated with testosterone and 74 patients had longitudinal Hgb, Hct, and T data. The majority (70, 95%) identified as male, were white/non-Hispanic (60, 81.1%), and had commercial insurance (58, 78.4%, Table 1). Target therapeutic range for T was 300-900 ng/dL, and the most common route of administration was subcutaneous injection. The mean baseline Hgb was 13.2 g/dL and Hct was 39.2%. The increase in Hgb and Hct was associated with increasing T after controlling for time (Figure 1, P < 0.01). Length of time on T correlated more with an increase in Hgb than maximum T at any given time point. One patient developed polycythemia (Hgb > 17.5 g/dL). Fifteen patients required dose adjustment based on significant Hgb/Hct increases from baseline, higher than desired T levels, or both. There were no negative health outcomes related to polycythemia including thrombosis.Conclusion(s): This study examines the rate of polycythemia in an adolescent transgender males on GAHT. A statistically significant association exists between the elevation of Hgb and Hct with T supplementation (Figure 1). Race, ethnicity, and BMI did not influence Hgb or Hct. Very few dose adjustments were required due to Hgb/Hct (10, 13.5%). Continued lab monitoring of the complete blood count in this patient population is needed; however, the risk of clinically significant sustained polycythemia is low. These data may serve to support clinical decisions on dose adjustments for adolescent transgender patients on testosterone.
Testosterone Effect on Hemoglobin and Hematocrit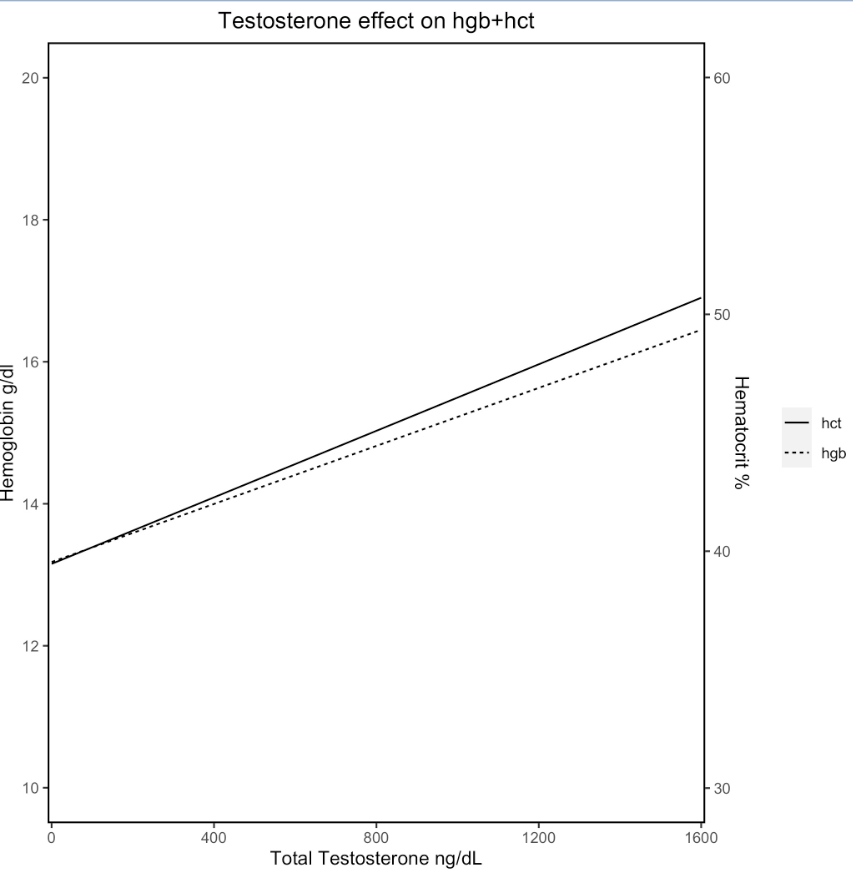 Figure 1: Fixed effect only regression line demonstrating the relationship between hemoglobin (Hgb), hematocrit (hct), and total testosterone under a mixed effect linear regression model when controlling for time (P < 0.01).
Figure 1: Fixed effect only regression line demonstrating the relationship between hemoglobin (Hgb), hematocrit (hct), and total testosterone under a mixed effect linear regression model when controlling for time (P < 0.01).
Table 1: Demographics of Study Population.png)
Objective: The study aim was to determine the rate of polycythemia for adolescent transgender male on GAHT with testosterone, clinical effects on this population, and risk of thrombosis.
Design/Methods: Patients seen in a Gender Wellness Program from 2015-2021 were studied. Included patients were started on testosterone therapy and data including hemoglobin (Hgb), hematocrit (Hct), and total serum testosterone (T) were collected at baseline and longitudinally. Data were analyzed using descriptive statistics and a linear mixed effect model.
Results: Of 189 patients, 104 were treated with testosterone and 74 patients had longitudinal Hgb, Hct, and T data. The majority (70, 95%) identified as male, were white/non-Hispanic (60, 81.1%), and had commercial insurance (58, 78.4%, Table 1). Target therapeutic range for T was 300-900 ng/dL, and the most common route of administration was subcutaneous injection. The mean baseline Hgb was 13.2 g/dL and Hct was 39.2%. The increase in Hgb and Hct was associated with increasing T after controlling for time (Figure 1, P < 0.01). Length of time on T correlated more with an increase in Hgb than maximum T at any given time point. One patient developed polycythemia (Hgb > 17.5 g/dL). Fifteen patients required dose adjustment based on significant Hgb/Hct increases from baseline, higher than desired T levels, or both. There were no negative health outcomes related to polycythemia including thrombosis.Conclusion(s): This study examines the rate of polycythemia in an adolescent transgender males on GAHT. A statistically significant association exists between the elevation of Hgb and Hct with T supplementation (Figure 1). Race, ethnicity, and BMI did not influence Hgb or Hct. Very few dose adjustments were required due to Hgb/Hct (10, 13.5%). Continued lab monitoring of the complete blood count in this patient population is needed; however, the risk of clinically significant sustained polycythemia is low. These data may serve to support clinical decisions on dose adjustments for adolescent transgender patients on testosterone.
Testosterone Effect on Hemoglobin and Hematocrit
 Figure 1: Fixed effect only regression line demonstrating the relationship between hemoglobin (Hgb), hematocrit (hct), and total testosterone under a mixed effect linear regression model when controlling for time (P < 0.01).
Figure 1: Fixed effect only regression line demonstrating the relationship between hemoglobin (Hgb), hematocrit (hct), and total testosterone under a mixed effect linear regression model when controlling for time (P < 0.01).Table 1: Demographics of Study Population
.png)
