Neonatal Neurology: Clinical
Category: Abstract Submission
Neurology 6: Neonatal Neurology Preterm Imaging
342 - Three-tissue decomposition of the neonatal brain using diffusion MRI
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 342
Publication Number: 342.442
Publication Number: 342.442
Manuel Blesa, University of Edinburgh, Edinburgh, Scotland, United Kingdom; Kadi Vaher, University of Edinburgh, Edinburgh, Scotland, United Kingdom; Paola Galdi, MRC Centre for Reproductive Health, University of Edinburgh, UK, Edinburgh, Scotland, United Kingdom; Gemma Sullivan, University of Edinburgh, Edinburgh, Scotland, United Kingdom; David Q. Stoye, National Health Service, St. Albans, England, United Kingdom; Jill Hall, University of Edinburgh, Edinburgh, Scotland, United Kingdom; Amy E. Corrigan, University of Edinburgh, Edinburgh, Scotland, United Kingdom; Alan J. Quigley, NHS Lothian, Edinburgh, Scotland, United Kingdom; Michael Thrippleton, University of Edinburgh, Edinburgh, Scotland, United Kingdom; Mark Bastin, University of Edinburgh, Edinburgh, Scotland, United Kingdom; Thijs Dhollander, Murdoch Children's Research Institute, Melbourne, Victoria, Australia; James P. Boardman, University of Edinburgh, Edinburgh, Scotland, United Kingdom

Manuel Blesa Cabez, PhD
Post Doctoral Fellow
University of Edinburgh
Edinburgh, Scotland, United Kingdom
Presenting Author(s)
Background: Preterm infants are at increased risk of alterations in brain structure and connectivity, and subsequent neurocognitive impairment. Diffusion MRI (dMRI), which is widely used to study preterm brain in vivo, has been instrumental for making inferences about microstructural alterations of white matter (WM) that characterize dysmaturity associated with preterm birth. Standard dMRI models (such as Diffusion Tensor Imaging) don’t include information about tissue properties in modelling. Three-tissue decomposition analysis is a quantitative method to study brain microstructure that incorporates information about tissue properties to provide biologically plausible information about brain microstructure; it has proven useful for characterising WM changes in Alzheimer's disease and stroke.
Objective: To assess quantitative, regional WM changes associated with preterm birth using 3-tissue decomposition
Design/Methods: 210 neonates underwent brain dMRI at term-equivalent age (Figure 1). dMRI volumes were processed using single-shell 3-tissue constrained spherical deconvolution, obtaining three maps per subject: cerebrospinal fluid (CSF)-like, grey matter (GM)-like and WM-like signal fractions (TC, TG and TW, respectively). A voxel-wise statistical comparison between groups was performed using GLM adjusting for gestational age at scan and correcting for multiple comparisons. In addition, we inspected the mean of each of the tissue fractions.
Results: Figure 2 shows the voxel-wise differences between term and preterm infants. The preterm group has higher values for TC and lower values for TW in most WM regions. Overall, within the WM mask the preterm group have a shift from TW towards the TC (Figure 3).Conclusion(s): These maps show the fraction of the signal that behaves as a standard CSF, GM or WM signal. The shift of the preterm group from TW to TC can be interpreted as a less mature WM, in which water moves more freely within WM and this is consistent with increased brain water content seen on conventional T1 and T2 weighted imaging. The contribution of TG might be compatible with an increased presence of glial cells, but this does not imply that such a region corresponds biologically to actual grey matter. More work needs to be done to investigate the potential confounding partial volume effects. The advantage of this method is that provides quantitative regional assessment of WM changes that can be understood in terms of tissue composition.
Figure 1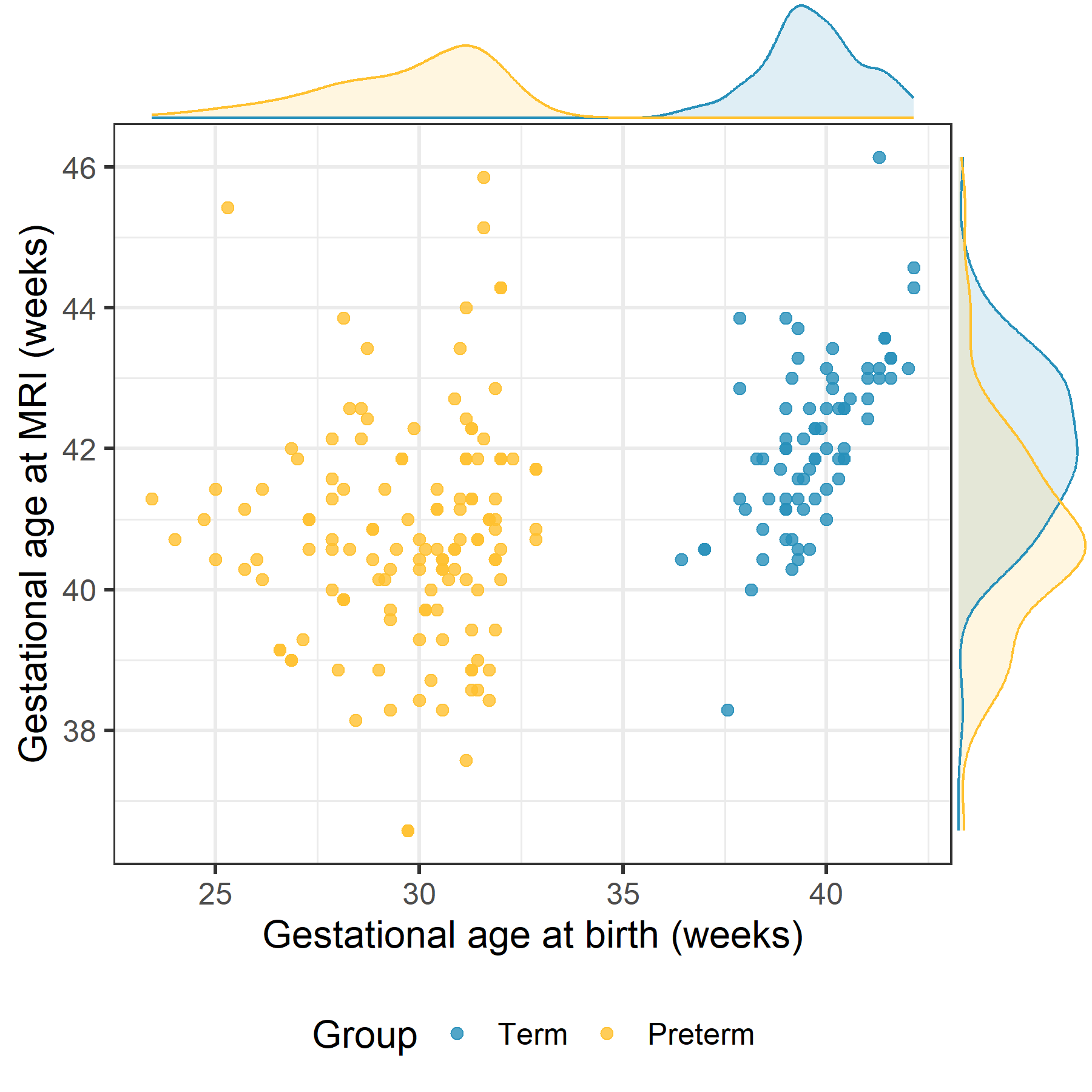 Gestational age (GA) at birth and at scan distribution of the preterm and term groups. 133 preterm (GA at birth = 29.7 ± 2.1 and GA at scan = 40.8 ± 1.6 weeks) and 77 term born preterm (GA at birth = 39.7 ± 1.2 and GA at scan = 42.1 ± 1.2 weeks).
Gestational age (GA) at birth and at scan distribution of the preterm and term groups. 133 preterm (GA at birth = 29.7 ± 2.1 and GA at scan = 40.8 ± 1.6 weeks) and 77 term born preterm (GA at birth = 39.7 ± 1.2 and GA at scan = 42.1 ± 1.2 weeks).
Figure 2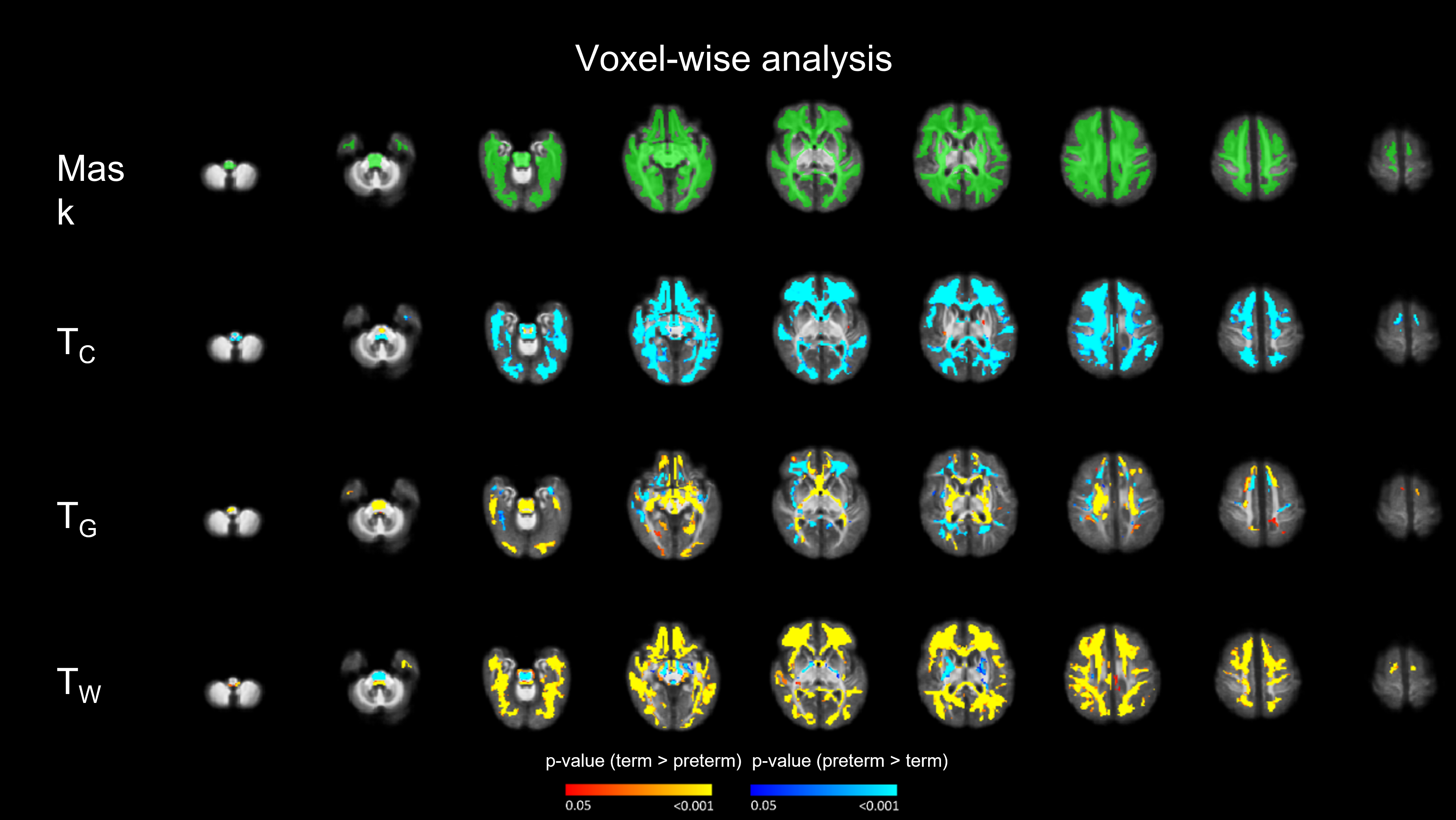 Voxel-wise analysis for the TC, TG and TW. Overall, the tendency is for the preterm infants to show a reduced TW and increased TC compared to the term controls. This reflects a more mature WM and reduced free water content in the term-born infants.
Voxel-wise analysis for the TC, TG and TW. Overall, the tendency is for the preterm infants to show a reduced TW and increased TC compared to the term controls. This reflects a more mature WM and reduced free water content in the term-born infants.
Objective: To assess quantitative, regional WM changes associated with preterm birth using 3-tissue decomposition
Design/Methods: 210 neonates underwent brain dMRI at term-equivalent age (Figure 1). dMRI volumes were processed using single-shell 3-tissue constrained spherical deconvolution, obtaining three maps per subject: cerebrospinal fluid (CSF)-like, grey matter (GM)-like and WM-like signal fractions (TC, TG and TW, respectively). A voxel-wise statistical comparison between groups was performed using GLM adjusting for gestational age at scan and correcting for multiple comparisons. In addition, we inspected the mean of each of the tissue fractions.
Results: Figure 2 shows the voxel-wise differences between term and preterm infants. The preterm group has higher values for TC and lower values for TW in most WM regions. Overall, within the WM mask the preterm group have a shift from TW towards the TC (Figure 3).Conclusion(s): These maps show the fraction of the signal that behaves as a standard CSF, GM or WM signal. The shift of the preterm group from TW to TC can be interpreted as a less mature WM, in which water moves more freely within WM and this is consistent with increased brain water content seen on conventional T1 and T2 weighted imaging. The contribution of TG might be compatible with an increased presence of glial cells, but this does not imply that such a region corresponds biologically to actual grey matter. More work needs to be done to investigate the potential confounding partial volume effects. The advantage of this method is that provides quantitative regional assessment of WM changes that can be understood in terms of tissue composition.
Figure 1
 Gestational age (GA) at birth and at scan distribution of the preterm and term groups. 133 preterm (GA at birth = 29.7 ± 2.1 and GA at scan = 40.8 ± 1.6 weeks) and 77 term born preterm (GA at birth = 39.7 ± 1.2 and GA at scan = 42.1 ± 1.2 weeks).
Gestational age (GA) at birth and at scan distribution of the preterm and term groups. 133 preterm (GA at birth = 29.7 ± 2.1 and GA at scan = 40.8 ± 1.6 weeks) and 77 term born preterm (GA at birth = 39.7 ± 1.2 and GA at scan = 42.1 ± 1.2 weeks).Figure 2
 Voxel-wise analysis for the TC, TG and TW. Overall, the tendency is for the preterm infants to show a reduced TW and increased TC compared to the term controls. This reflects a more mature WM and reduced free water content in the term-born infants.
Voxel-wise analysis for the TC, TG and TW. Overall, the tendency is for the preterm infants to show a reduced TW and increased TC compared to the term controls. This reflects a more mature WM and reduced free water content in the term-born infants.