Neonatal Pulmonology
Category: Abstract Submission
Neonatal Pulmonology IV: Lung Cellular Molecular Biology and Biomarkers
463 - Hyperoxic exposure leads to senescence in lung epithelial cell via increased microRNA-34a
Monday, April 25, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 463
Publication Number: 463.432
Publication Number: 463.432
Hajime Maeda, Brown University, Providence, RI, United States; Hongwei Yao, Brown University, Providence, RI, United States; Daniella Teape, The Warren Alpert Medical School of Brown University, providence, RI, United States; Phyllis Dennery, The Warren Alpert Medical School of Brown University, Providence, RI, United States

Hajime Maeda, MD
Research Associate
Brown University
Providence, Rhode Island, United States
Presenting Author(s)
Background: Bronchopulmonary dysplasia (BPD) is a chronic lung disease in premature infants, characterized by alveolar dysplasia and impaired vascularization. Supplemental oxygen and mechanical ventilation, commonly used in premature infants, may result in BPD. We and others have shown that exposure to high concentration of oxygen (hyperoxia) causes permanent proliferation arrest (senescence) in lung fibroblasts, epithelial and smooth muscle cells. Previous studies have shown that miR-34a is increased in the lung of patients with BPD and hyperoxia-exposed mice. Although miR-34a participates in cellular senescence, it is unclear whether it contributes to hyperoxia-induced senescence.
Objective: To investigate whether hyperoxia increases miR-34a levels, leading to cellular senescence.
Design/Methods: Mouse lung epithelial cells (MLE-12) and primary human small airway epithelial cells (SAEC) were exposed to hyperoxia (95% O2/5% CO2) or air (21% O2/5% CO2) for 24 h. Newborn mice ( < 12 h old) were also exposed to hyperoxia (>95% O2) for 3 days and allowed to recover in room air until postnatal days (PND) 7. We evaluated miR-34a and senescence biomarkers, including nuclear lamin B1 exclusion, p53 and p21 gene expression, in the cultured cells and mouse lungs. Furthermore, we evaluated hyperoxia-induced senescence in MLE-12 cells after transfection with an miR-34a inhibitor (20nM) or mimic (0.1, 1 and 10nM).
Results: Hyperoxia resulted in senescence in MLE-12 and SAEC cells, as indicated by increased nuclear lamin B1 exclusion, p21 and p53 mRNA. Lung p21 mRNA was also increased at PND3 and lamin B1 exclusion was increased at PND7 in mice exposed to hyperoxia as neonates. Hyperoxic exposure significantly increased miR-34a expression in hyperoxia-exposed cells (1.6-fold) and in the lung of neonatal mice exposed to hyperoxia (2.6-fold and 1.7-fold at PND3 and PND7, respectively). Transfection of the miR-34a inhibitor suppressed miR-34a expression and reduced all hyperoxia induced markers of senescence in MLE-12 cells. Transfection with a miR-34a mimic increased miR-34a levels in a concentration-dependent manner. However, miR-34a mimic transfection (0.1nM or 1nM) did not increase p21 or p53 in MLE-12 cells with hyperoxic exposure.Conclusion(s): Hyperoxia increases miR-34a, leading to lung epithelial cell senescence. Therefore, preventing hyperoxia-induced senescence via the suppression of miR-34a may provide a novel therapeutic strategy to mitigate the detrimental consequences of hyperoxia in the neonatal lung.
Hyperoxia increased nuclear lamin B1 exclusion in MLE-12 cells.jpg)
Hyperoxia increased p53 and p21 mRNA in MLE-12 cells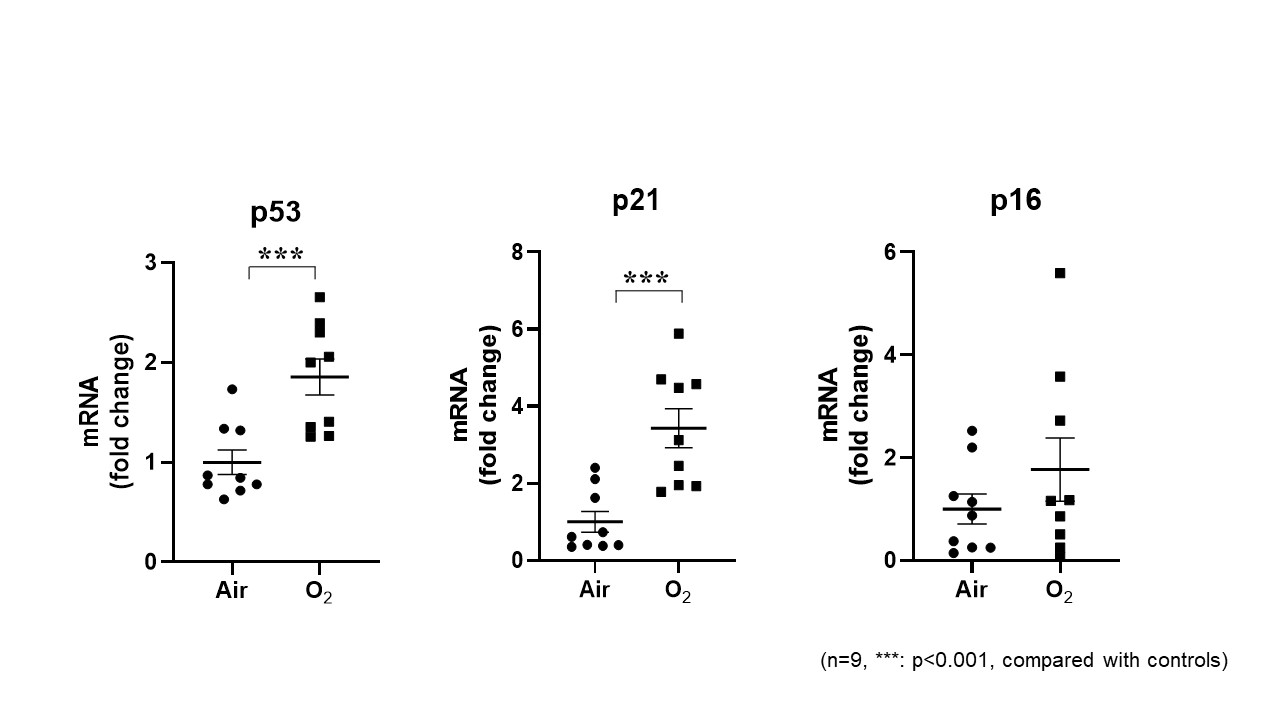
Objective: To investigate whether hyperoxia increases miR-34a levels, leading to cellular senescence.
Design/Methods: Mouse lung epithelial cells (MLE-12) and primary human small airway epithelial cells (SAEC) were exposed to hyperoxia (95% O2/5% CO2) or air (21% O2/5% CO2) for 24 h. Newborn mice ( < 12 h old) were also exposed to hyperoxia (>95% O2) for 3 days and allowed to recover in room air until postnatal days (PND) 7. We evaluated miR-34a and senescence biomarkers, including nuclear lamin B1 exclusion, p53 and p21 gene expression, in the cultured cells and mouse lungs. Furthermore, we evaluated hyperoxia-induced senescence in MLE-12 cells after transfection with an miR-34a inhibitor (20nM) or mimic (0.1, 1 and 10nM).
Results: Hyperoxia resulted in senescence in MLE-12 and SAEC cells, as indicated by increased nuclear lamin B1 exclusion, p21 and p53 mRNA. Lung p21 mRNA was also increased at PND3 and lamin B1 exclusion was increased at PND7 in mice exposed to hyperoxia as neonates. Hyperoxic exposure significantly increased miR-34a expression in hyperoxia-exposed cells (1.6-fold) and in the lung of neonatal mice exposed to hyperoxia (2.6-fold and 1.7-fold at PND3 and PND7, respectively). Transfection of the miR-34a inhibitor suppressed miR-34a expression and reduced all hyperoxia induced markers of senescence in MLE-12 cells. Transfection with a miR-34a mimic increased miR-34a levels in a concentration-dependent manner. However, miR-34a mimic transfection (0.1nM or 1nM) did not increase p21 or p53 in MLE-12 cells with hyperoxic exposure.Conclusion(s): Hyperoxia increases miR-34a, leading to lung epithelial cell senescence. Therefore, preventing hyperoxia-induced senescence via the suppression of miR-34a may provide a novel therapeutic strategy to mitigate the detrimental consequences of hyperoxia in the neonatal lung.
Hyperoxia increased nuclear lamin B1 exclusion in MLE-12 cells
.jpg)
Hyperoxia increased p53 and p21 mRNA in MLE-12 cells

