Critical Care
Category: Abstract Submission
Critical Care III
100 - Adrenal Insufficiency After Glucocorticoid Use in the Pediatric Intensive Care Unit
Sunday, April 24, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 100
Publication Number: 100.307
Publication Number: 100.307
Ashley N. Radig, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, IA, United States; Vanessa A. Curtis, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, IA, United States; Christina L. Cifra, University of Iowa Roy J. and Lucille A. Carver College of Medicine, Iowa City, IA, United States

Ashley N. Radig, B.A.
Medical Student
University of Iowa Roy J. and Lucille A. Carver College of Medicine
Iowa City, Iowa, United States
Presenting Author(s)
Background: Glucocorticoids are commonly used in the pediatric intensive care unit (PICU) for a variety of indications. Adrenal insufficiency (AI) secondary to glucocorticoid (GC) use among critically ill children has not been well-characterized despite its potential impact on post-critical illness morbidity and recovery.
Objective: Determine the frequency of subsequent AI among critically ill children who received GCs during their PICU admission.
Design/Methods: We conducted a retrospective cohort study using structured medical record review. We included patients 0-18 yrs consecutively admitted to a single tertiary referral PICU over 2 yrs who received enteral/parenteral GCs (dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and/or prednisone) during their PICU admission. We excluded patients with known AI or HPA disease prior to GC administration in the PICU and patients with subsequent PICU admissions involving GC use. We determined subsequent AI diagnosis within 1 yr of PICU admission if one or more of the following were documented: morning cortisol < 10 mcg/dL, ACTH stimulation test with cortisol < 18 mcg/dL, clinician’s diagnosis of AI indicated in a clinical note.
Results: We identified 530 patients who received GCs during the study period, of which 12 (2.3%) were diagnosed with AI at a median of 55 (IQR 8-156) days after GC first use in the PICU. Unadjusted analysis showed that patients who subsequently developed AI were younger (median 0.5 vs. 2 yrs, p=0.020). There were no significant differences in sex, race/ethnicity, illness severity, and diagnostic category (Table 1). Patients with AI were more likely to have received GCs for hyperinflammation (20.7% vs. 7.7%) and less likely for asthma/reactive airways (10.3% vs. 26.3%) (p=0.0.36), had a higher median total daily hydrocortisone equivalent dose (2508 vs. 480 mg, p=0.007), had a longer median total duration of GC use (12 vs. 4 d, p < 0.001), and were more likely to have had a steroid taper (48.3% vs. 23.6%, p=0.003) (Table 2). Patients with AI had longer median PICU (78.5 vs. 4 d, p < 0.001) and hospital (96 vs. 6 d, p < 0.001) stays and were less likely to survive to 1 yr after PICU discharge (75% vs. 94.2%, p=0.033).Conclusion(s): 2.3% of PICU patients developed AI after GC use for critical illness. We identified risk factors for AI after GC use, which are important to recognize due to lower survival of PICU patients with AI. Future work is needed to delineate how AI contributes to morbidity and mortality in critically ill children and how to mitigate this effect.
Table 1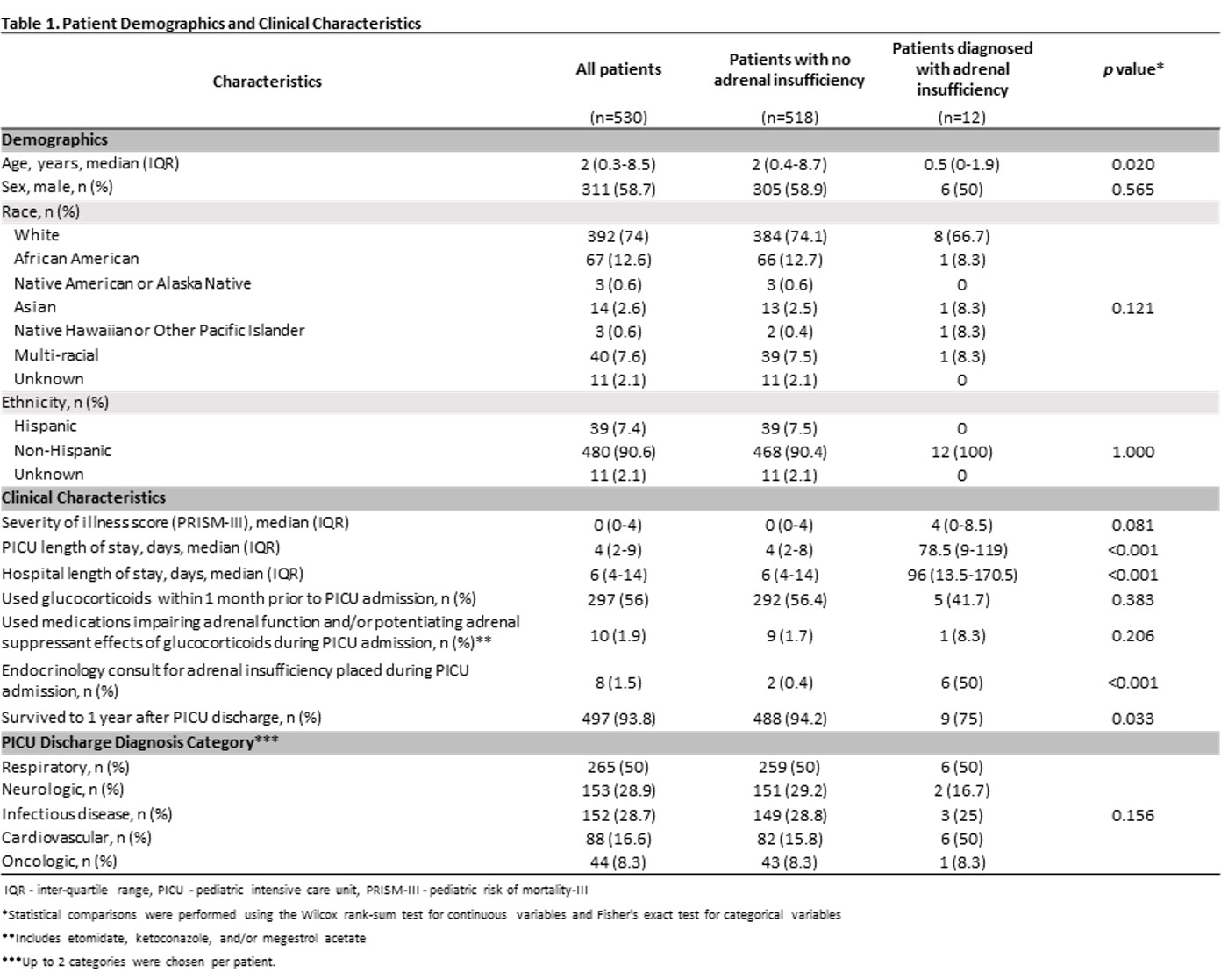 Patient Demographics and Clinical Characteristics
Patient Demographics and Clinical Characteristics
Table 2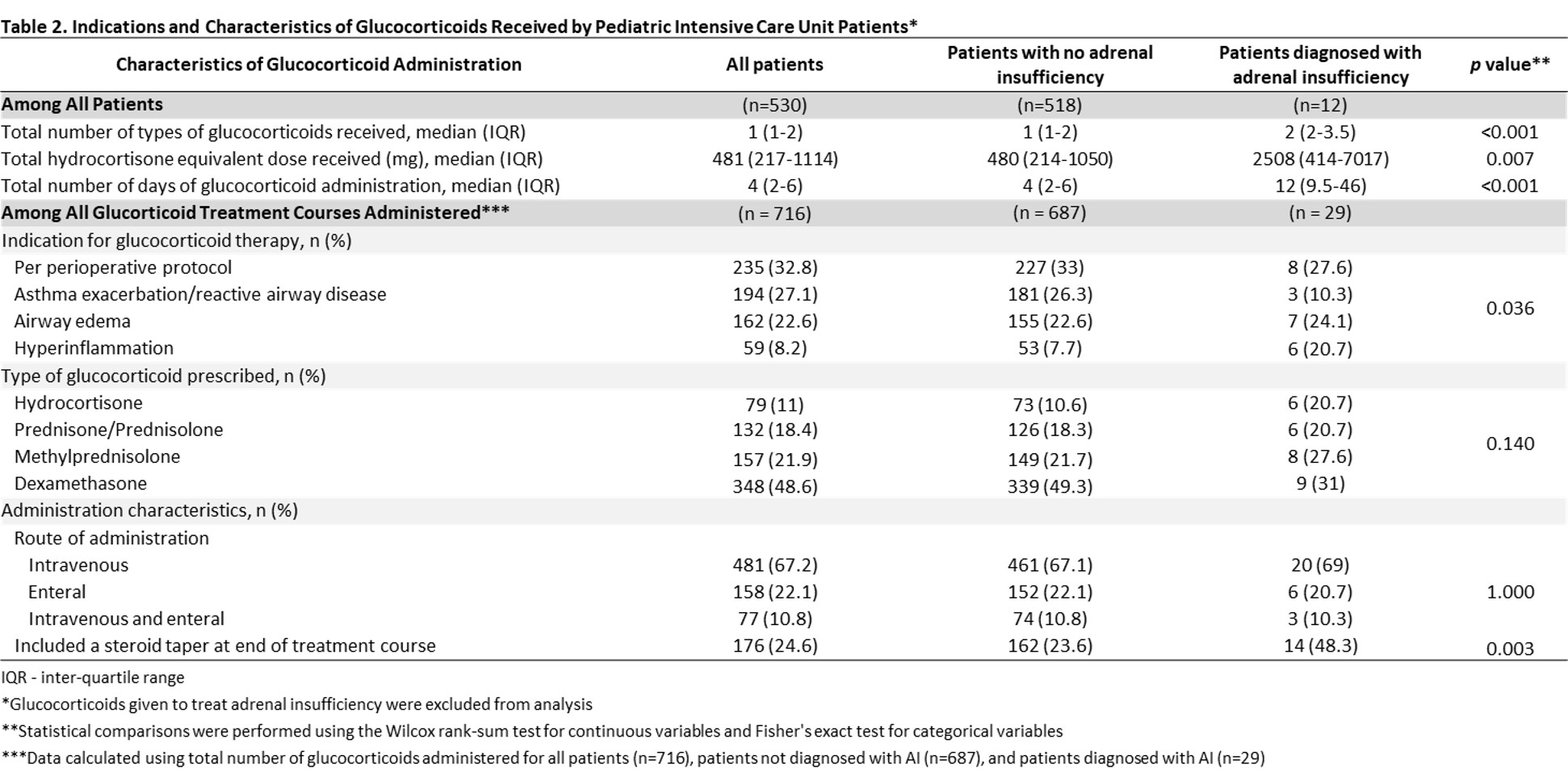 Indications and Characteristics of Glucocorticoids Received by Pediatric Intensive Care Unit Patients
Indications and Characteristics of Glucocorticoids Received by Pediatric Intensive Care Unit Patients
Objective: Determine the frequency of subsequent AI among critically ill children who received GCs during their PICU admission.
Design/Methods: We conducted a retrospective cohort study using structured medical record review. We included patients 0-18 yrs consecutively admitted to a single tertiary referral PICU over 2 yrs who received enteral/parenteral GCs (dexamethasone, hydrocortisone, methylprednisolone, prednisolone, and/or prednisone) during their PICU admission. We excluded patients with known AI or HPA disease prior to GC administration in the PICU and patients with subsequent PICU admissions involving GC use. We determined subsequent AI diagnosis within 1 yr of PICU admission if one or more of the following were documented: morning cortisol < 10 mcg/dL, ACTH stimulation test with cortisol < 18 mcg/dL, clinician’s diagnosis of AI indicated in a clinical note.
Results: We identified 530 patients who received GCs during the study period, of which 12 (2.3%) were diagnosed with AI at a median of 55 (IQR 8-156) days after GC first use in the PICU. Unadjusted analysis showed that patients who subsequently developed AI were younger (median 0.5 vs. 2 yrs, p=0.020). There were no significant differences in sex, race/ethnicity, illness severity, and diagnostic category (Table 1). Patients with AI were more likely to have received GCs for hyperinflammation (20.7% vs. 7.7%) and less likely for asthma/reactive airways (10.3% vs. 26.3%) (p=0.0.36), had a higher median total daily hydrocortisone equivalent dose (2508 vs. 480 mg, p=0.007), had a longer median total duration of GC use (12 vs. 4 d, p < 0.001), and were more likely to have had a steroid taper (48.3% vs. 23.6%, p=0.003) (Table 2). Patients with AI had longer median PICU (78.5 vs. 4 d, p < 0.001) and hospital (96 vs. 6 d, p < 0.001) stays and were less likely to survive to 1 yr after PICU discharge (75% vs. 94.2%, p=0.033).Conclusion(s): 2.3% of PICU patients developed AI after GC use for critical illness. We identified risk factors for AI after GC use, which are important to recognize due to lower survival of PICU patients with AI. Future work is needed to delineate how AI contributes to morbidity and mortality in critically ill children and how to mitigate this effect.
Table 1
 Patient Demographics and Clinical Characteristics
Patient Demographics and Clinical CharacteristicsTable 2
 Indications and Characteristics of Glucocorticoids Received by Pediatric Intensive Care Unit Patients
Indications and Characteristics of Glucocorticoids Received by Pediatric Intensive Care Unit Patients