Hospital Medicine: Clinical
Category: Abstract Submission
Hospital Medicine: Clinical NOS
330 - Safety Data After Implementation of a Pragmatic Trial Using a Predictive Model for Venous Thromboembolism (VTE) Prevention
Saturday, April 23, 2022
3:30 PM - 6:00 PM US MT
Poster Number: 330
Publication Number: 330.214
Publication Number: 330.214
Shannon C. Walker, Vanderbilt University Medical Center, Nashville, TN, United States; Benjamin French, Vanderbilt University Medical Center, Nashville, TN, United States; Ryan Moore, Vanderbilt University School of Medicine, Nashville, TN, United States; Henry J. Domenico, Vanderbilt University Medical Center, Nashville, TN, United States; C. Buddy Creech, Vanderbilt University School of Medicine, Nashville, TN, United States; Daniel W. Byrne, Vanderbilt University School of Medicine, Charlottesville, VA, United States; Allison P. Wheeler, Vanderbilt University Medical Center, Brentwood, TN, United States

Shannon C. Walker, MD
Clinical Fellow - Transfusion Medicine
Vanderbilt University Medical Center
Nashville, Tennessee, United States
Presenting Author(s)
Background: Hospital-acquired venous thromboembolism (HA-VTE) is a significant cause of morbidity and mortality in pediatric populations. Unfortunately, identification of pediatric patients at increased risk who may benefit from prophylactic anticoagulation remains challenging; furthermore, risk stratification should be as specific as possible, since anticoagulation places patients at risk for bleeding, can require laboratory safety monitoring, and typically involves injections. Given these challenges, it is essential to develop a personalized approach to identify children at highest risk for VTE. We developed and validated a general pediatric real-time risk prediction model from a large, single center cohort, using 111,352 admissions in the derivation cohort and 44,138 admissions in a separate validation cohort, which we are now assessing along with targeted intervention in a pragmatic randomized trial.
Objective: We report on the safety of patients in the ongoing trial, with data from November 2, 2020 through October 31, 2021.
Design/Methods: All patients admitted to Monroe Carell Jr. Children’s Hospital at Vanderbilt who are 21 years of age and younger are included in the study. The automated EMR-based prediction model calculates the predicted probability of HA-VTE for all patients. Figure 1 displays the study schema. The primary endpoint of the study is the frequency of HA-VTE events per arm, and secondary endpoints include the number of patients in the intervention arm who receive anticoagulation and the number of bleeding-related adverse events. Bleeding-related adverse events are graded using the modified WHO bleeding scale (Figure 2).
Results: From November 2, 2020 through October 31, 2021, there were 14,453 eligible hospital admissions. 389 admissions in the intervention arm had an elevated risk of developing HA-VTE (predicted probability >0.025). These were reviewed by the hematology research team and recommendations were made to the clinical team. Prophylactic anticoagulation was recommended for 253 of these admissions and was initiated on 71 admissions. One patient developed a grade I bleeding event (oropharyngeal bleeding) and two patients developed Grade II bleeding events (hemoptysis and bleeding at procedure site). No patients developed grade III or grade IV bleeding events. Figure 3 displays this in detail.Conclusion(s): The use of validated prognostic model, coupled with hematology review for patients at elevated risk for developing HA-VTE, is safe and does not increase the risk of severe bleeding in patients started on prophylactic anticoagulation.
CV: Shannon C. WalkerCV 2022 SCW .pdf
Figure 2: Modified WHO Bleeding Grades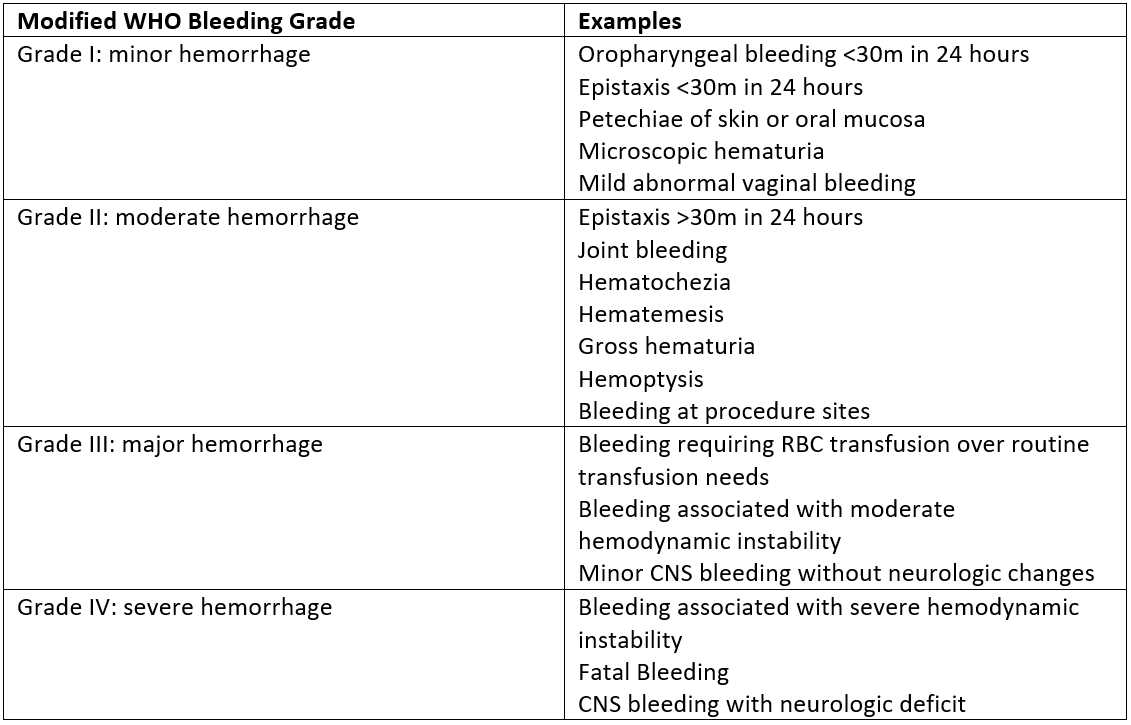 The figure describes the modified WHO bleeding grades used in the study to quantify bleeding episodes.
The figure describes the modified WHO bleeding grades used in the study to quantify bleeding episodes.
Objective: We report on the safety of patients in the ongoing trial, with data from November 2, 2020 through October 31, 2021.
Design/Methods: All patients admitted to Monroe Carell Jr. Children’s Hospital at Vanderbilt who are 21 years of age and younger are included in the study. The automated EMR-based prediction model calculates the predicted probability of HA-VTE for all patients. Figure 1 displays the study schema. The primary endpoint of the study is the frequency of HA-VTE events per arm, and secondary endpoints include the number of patients in the intervention arm who receive anticoagulation and the number of bleeding-related adverse events. Bleeding-related adverse events are graded using the modified WHO bleeding scale (Figure 2).
Results: From November 2, 2020 through October 31, 2021, there were 14,453 eligible hospital admissions. 389 admissions in the intervention arm had an elevated risk of developing HA-VTE (predicted probability >0.025). These were reviewed by the hematology research team and recommendations were made to the clinical team. Prophylactic anticoagulation was recommended for 253 of these admissions and was initiated on 71 admissions. One patient developed a grade I bleeding event (oropharyngeal bleeding) and two patients developed Grade II bleeding events (hemoptysis and bleeding at procedure site). No patients developed grade III or grade IV bleeding events. Figure 3 displays this in detail.Conclusion(s): The use of validated prognostic model, coupled with hematology review for patients at elevated risk for developing HA-VTE, is safe and does not increase the risk of severe bleeding in patients started on prophylactic anticoagulation.
CV: Shannon C. WalkerCV 2022 SCW .pdf
Figure 2: Modified WHO Bleeding Grades
 The figure describes the modified WHO bleeding grades used in the study to quantify bleeding episodes.
The figure describes the modified WHO bleeding grades used in the study to quantify bleeding episodes.